Beta decay is a type of radioactive decay in which an unstable atomic nucleus transforms by emitting a beta particle, which can be an electron or a positron. This process changes the composition of the nucleus and alters its atomic structure, often resulting in a different element. Beta decay is one of the three main types of radioactive decay, alongside alpha decay and gamma decay. It plays a significant role in nuclear physics, astrophysics, and medical imaging.
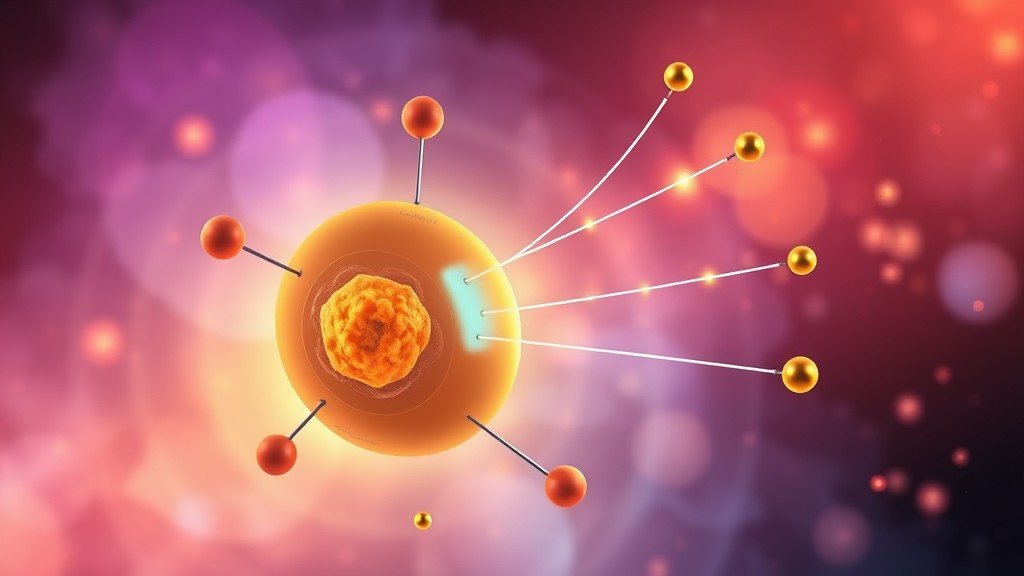
In this article, we will explore the concept of beta decay, the types of beta decay, the mechanism by which it occurs, and practical applications, with examples to clarify each concept.
What is Beta Decay?
Beta decay is a process by which certain unstable nuclei release excess energy by emitting a beta particle. This emission occurs to achieve a more stable nuclear configuration. A beta particle can either be an electron (![]() ) or a positron (
) or a positron (![]() ), depending on the type of beta decay. During beta decay, the atomic nucleus changes its neutron or proton composition, resulting in the transformation of one element into another.
), depending on the type of beta decay. During beta decay, the atomic nucleus changes its neutron or proton composition, resulting in the transformation of one element into another.
Types of Beta Decay
There are two primary types of beta decay, each involving a different kind of beta particle:
1. Beta-minus (β⁻) Decay: In beta-minus decay, a neutron transforms into a proton, emitting an electron and an antineutrino.
2. Beta-plus (β⁺) Decay: In beta-plus decay, a proton transforms into a neutron, emitting a positron and a neutrino.
Each type of beta decay has a distinct mechanism and outcome, which we’ll examine in detail below.
Mechanism of Beta Decay
Beta decay occurs when there is an imbalance in the proton-to-neutron ratio within an atomic nucleus. In an effort to achieve stability, the nucleus undergoes transformation by changing a neutron into a proton or vice versa, accompanied by the emission of beta particles and neutrinos.
1. Beta-minus (β⁻) Decay
Beta-minus decay occurs in neutron-rich nuclei, where there are more neutrons than protons. In this process:
- A neutron (
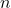 ) in the nucleus transforms into a proton (
) in the nucleus transforms into a proton (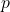 ).
). - An electron (beta-minus particle,
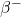 ) and an antineutrino (
) and an antineutrino (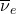 ) are emitted.
) are emitted. - This decay increases the atomic number by one, transforming the nucleus into a different element.
The equation for beta-minus decay can be represented as follows:
![]()
or, for an example nucleus:
![]()
In this example, a carbon-14 (![]() ) nucleus decays into a nitrogen-14 (
) nucleus decays into a nitrogen-14 (![]() ) nucleus, emitting a beta particle (electron) and an antineutrino.
) nucleus, emitting a beta particle (electron) and an antineutrino.
2. Beta-plus (β⁺) Decay
Beta-plus decay occurs in proton-rich nuclei, where there are more protons than neutrons. In this process:
- A proton (
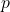 ) in the nucleus transforms into a neutron (
) in the nucleus transforms into a neutron (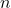 ).
). - A positron (beta-plus particle,
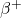 ) and a neutrino (
) and a neutrino (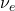 ) are emitted.
) are emitted. - This decay decreases the atomic number by one, resulting in the transformation of the nucleus into a different element.
The equation for beta-plus decay can be represented as follows:
![]()
or, for an example nucleus:
![]()
In this example, a carbon-11 (![]() ) nucleus decays into a boron-11 (
) nucleus decays into a boron-11 (![]() ) nucleus, emitting a positron and a neutrino.
) nucleus, emitting a positron and a neutrino.
Neutrino and Antineutrino Emission
In both beta-minus and beta-plus decay, the emission of neutrinos (or antineutrinos) is essential. Neutrinos are nearly massless particles with no electric charge and interact very weakly with matter. The emission of these particles conserves certain fundamental properties, such as energy, momentum, and lepton number, in the decay process. This conservation ensures that beta decay obeys the laws of physics.
Energy Distribution in Beta Decay
The energy released during beta decay is shared between the beta particle (electron or positron) and the neutrino or antineutrino. The energy distribution is continuous because the emitted particles share the available energy in varying proportions. This continuous energy spectrum is one of the unique characteristics of beta decay, distinguishing it from alpha decay, which has a discrete energy spectrum.
Properties of Beta Particles
Beta particles, whether electrons or positrons, have specific characteristics that influence their behavior and applications.
1. Penetrating Power: Beta particles are more penetrating than alpha particles but less than gamma rays. They can travel a few millimeters through soft tissue and can be stopped by materials like plastic or aluminum.
2. Charge and Mass: Beta-minus particles are negatively charged electrons, while beta-plus particles are positively charged positrons. Both types have a very small mass relative to protons and neutrons.
3. Ionizing Power: Beta particles have moderate ionizing power. They can ionize atoms in their path, which makes them useful in applications like medical imaging and radiation therapy.
Applications of Beta Decay
Beta decay has a wide range of applications in various fields, from medical imaging and radiation therapy to radioactive dating and nuclear reactors. Below are some notable applications that demonstrate the practical significance of beta decay.
1. Medical Imaging and Positron Emission Tomography (PET)
In medical imaging, beta-plus decay is used in Positron Emission Tomography (PET) scans. PET scans are diagnostic tools that provide detailed images of organs and tissues by detecting gamma rays emitted when positrons (beta-plus particles) annihilate with electrons in the body.
Example: In PET imaging, a patient is injected with a radioactive tracer, such as fluorine-18 (![]() ), which undergoes beta-plus decay to produce positrons. These positrons interact with electrons in the body, producing gamma rays that are detected by the PET scanner, creating a detailed image of metabolic activity.
), which undergoes beta-plus decay to produce positrons. These positrons interact with electrons in the body, producing gamma rays that are detected by the PET scanner, creating a detailed image of metabolic activity.
![]()
2. Radiation Therapy for Cancer Treatment
Beta-minus particles emitted during beta decay are used in certain types of radiation therapy to target cancer cells. In this treatment, beta-emitting radioisotopes are introduced near or within cancerous tissue, where beta particles damage and kill the cancer cells.
Example: Strontium-90 (![]() ) is a beta-emitting isotope used in radiation therapy. It emits beta particles that deliver a controlled dose of radiation to kill cancerous cells without damaging surrounding healthy tissue.
) is a beta-emitting isotope used in radiation therapy. It emits beta particles that deliver a controlled dose of radiation to kill cancerous cells without damaging surrounding healthy tissue.
![]()
3. Carbon Dating and Radioactive Dating
Beta decay is the principle behind carbon dating, a technique used to determine the age of ancient organic materials. Carbon-14 (![]() ), a radioactive isotope, undergoes beta-minus decay with a known half-life, enabling scientists to calculate the time since the death of an organism.
), a radioactive isotope, undergoes beta-minus decay with a known half-life, enabling scientists to calculate the time since the death of an organism.
Example: When an organism dies, the intake of carbon-14 stops, and the carbon-14 present in the organism starts to decay into nitrogen-14 (![]() ). By measuring the remaining carbon-14 in a sample, scientists can estimate the time since the organism’s death.
). By measuring the remaining carbon-14 in a sample, scientists can estimate the time since the organism’s death.
![]()
4. Power Generation in Radioisotope Thermoelectric Generators (RTGs)
Beta decay is utilized in Radioisotope Thermoelectric Generators (RTGs), which are devices that provide power to spacecraft by converting heat from radioactive decay into electricity. RTGs rely on beta-emitting isotopes, such as strontium-90 and promethium-147, to generate heat that is then converted into electric power.
Example: Spacecraft, including those on long-duration missions, use RTGs for power, enabling them to function in environments far from the sun where solar power is impractical.
5. Applications in Scientific Research
Beta decay is used to study particle physics and fundamental interactions. The study of beta particles and neutrinos in beta decay has led to discoveries about weak nuclear forces and has provided insights into the behavior of subatomic particles.
Example: Experiments with beta decay in laboratories help physicists understand neutrino properties, as neutrinos are produced in beta decay processes. This research contributes to a broader understanding of the universe’s fundamental particles and forces.
Beta Decay and Safety Considerations
While beta particles are less penetrating than gamma rays, they still pose health risks if not handled carefully. Exposure to beta-emitting isotopes can
result in skin burns and damage to tissues, especially if the isotopes are inhaled or ingested. Proper safety measures, including the use of shielding materials like plastic and minimizing direct exposure, are necessary when working with beta emitters.
Shielding and Protection
1. Plastic and Aluminum: Since beta particles can penetrate several millimeters of tissue but are blocked by materials like plastic and aluminum, these materials are commonly used to shield beta sources.
2. Distance and Minimizing Exposure: Keeping a safe distance from beta-emitting sources and limiting exposure time are important safety measures.
3. Handling in Closed Containers: Beta-emitting isotopes are handled in closed containers or shielded environments in laboratories and hospitals to prevent contamination and exposure.
Differences Between Beta Decay and Other Radioactive Decay Types
Beta decay differs from alpha and gamma decay in both its mechanism and particles emitted:
1. Alpha Decay: In alpha decay, a helium-4 nucleus (alpha particle) is emitted from the nucleus. This process reduces the atomic mass and atomic number of the nucleus, and alpha particles are highly ionizing but have low penetration power.
2. Gamma Decay: Gamma decay involves the emission of gamma rays, which are high-energy photons, from an excited nucleus. Unlike beta and alpha decay, gamma decay does not alter the number of protons or neutrons in the nucleus; it only releases excess energy.
Conclusion
Beta decay is a significant form of radioactive decay in which an unstable nucleus releases an electron or positron to achieve stability. By changing a neutron into a proton or a proton into a neutron, beta decay alters the atomic composition, often resulting in a transformation into a different element. Beta decay’s role in medical imaging, cancer treatment, radioactive dating, and scientific research highlights its importance across multiple fields. Through the emission of beta particles and neutrinos, beta decay helps us understand atomic structure, subatomic particles, and fundamental forces, contributing to both technological advancements and scientific knowledge.