Electricity can be conducted through various states of matter, including solids, liquids, and gases. In liquids, the conduction of electricity is particularly fascinating, as it differs significantly from conduction in metals or other solid conductors. While conduction in solids relies on the movement of electrons, conduction in liquids depends primarily on the movement of ions. This process is essential in various fields, from electrochemistry and industrial applications to biological systems.
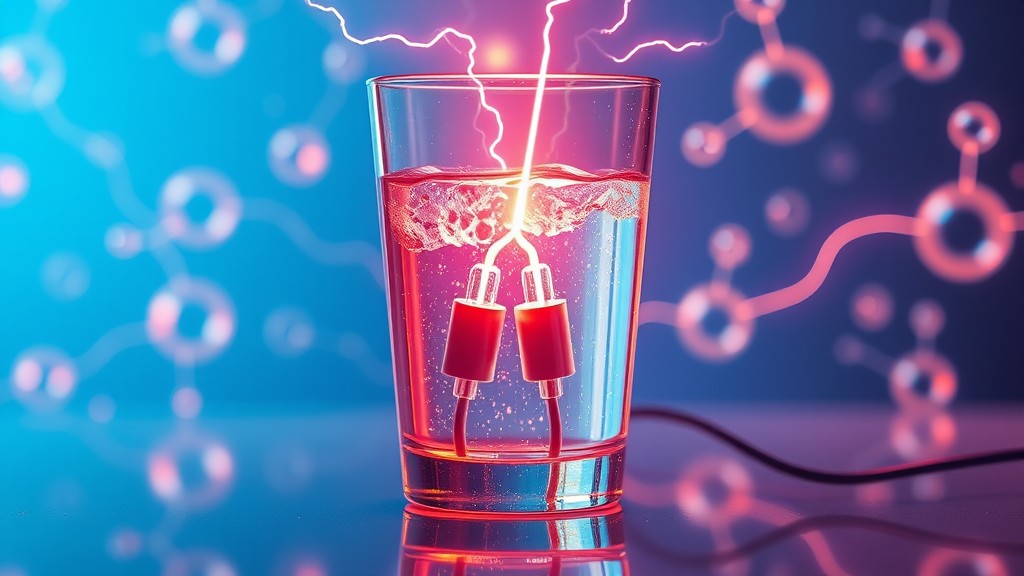
This article delves into the concept of electrical conduction in liquids, the mechanisms involved, factors affecting conductivity, and practical applications that highlight its importance in both science and industry.
What is Electrical Conduction in Liquids?
Electrical conduction in liquids occurs when an electric current flows through a liquid medium, facilitated by the movement of ions within the liquid. Unlike metals, where conduction is due to the free movement of electrons, the conduction process in liquids relies on positively and negatively charged ions, which are atoms or molecules that have lost or gained electrons, respectively. For a liquid to conduct electricity, it must contain a sufficient concentration of these charged ions.
Electrolytes: The Conduction-Enabled Liquids
Liquids that conduct electricity are called *electrolytes*. An electrolyte is a liquid, often a solution of salts, acids, or bases, that contains ions. These ions enable the transfer of electric charge, making the solution conductive. Common examples of electrolytes include saltwater (sodium chloride dissolved in water), sulfuric acid, and aqueous solutions of potassium chloride.
1. Strong Electrolytes: Strong electrolytes completely dissociate into ions in solution, resulting in high conductivity. Examples include hydrochloric acid (HCl) and sodium chloride (NaCl) dissolved in water.
2. Weak Electrolytes: Weak electrolytes partially dissociate, resulting in lower ion concentrations and, consequently, lower conductivity. Examples include acetic acid (CH₃COOH) and ammonium hydroxide (NH₄OH).
3. Non-Electrolytes: Non-electrolytes do not dissociate into ions and thus do not conduct electricity. Examples include pure distilled water, glucose solution, and most organic solvents like alcohol.
Mechanism of Electrical Conduction in Liquids
In liquids, electrical conduction occurs through the movement of ions rather than electrons. When an electric potential (voltage) is applied across an electrolyte, the positively charged ions (cations) migrate toward the negatively charged electrode (cathode), and the negatively charged ions (anions) move toward the positively charged electrode (anode). This movement of ions constitutes an electric current.
1. Ionization: When a substance like salt is dissolved in water, it dissociates into positive and negative ions, forming an electrolyte. For example, sodium chloride (NaCl) dissociates into Na⁺ and Cl⁻ ions in water.
2. Migration of Ions: Upon applying a voltage across the electrolyte, ions move through the solution. Cations are attracted to the cathode, while anions are attracted to the anode.
3. Electric Current: The movement of ions in opposite directions within the liquid medium completes the circuit, allowing electric current to flow through the liquid.
Example of Conduction in an Electrolyte Solution
Consider a beaker containing saltwater. When a battery is connected with electrodes dipped into the solution, sodium ions (Na⁺) move toward the cathode, and chloride ions (Cl⁻) move toward the anode. The movement of these ions in opposite directions facilitates the flow of current through the solution, making saltwater a good conductor of electricity.
Factors Affecting the Conduction of Electricity in Liquids
The ability of a liquid to conduct electricity depends on several factors, including ion concentration, temperature, the nature of the liquid, and the type of ions present. Let’s explore each of these factors and understand how they influence the conductivity of liquids.
1. Concentration of Ions
The concentration of ions in a liquid is directly related to its conductivity. A higher concentration of ions means there are more charge carriers available, resulting in higher conductivity.
Example: A saturated saltwater solution has a higher concentration of sodium (Na⁺) and chloride (Cl⁻) ions compared to a dilute solution. As a result, saturated saltwater is more conductive than a dilute saltwater solution.
2. Temperature
Temperature plays a significant role in the conduction of electricity in liquids. As temperature increases, the kinetic energy of ions also increases, leading to faster movement and higher conductivity. Higher temperatures reduce the viscosity of the liquid, allowing ions to move more freely.
Example: When warm water is used as an electrolyte, it conducts electricity better than cold water due to increased ion mobility at higher temperatures.
3. Nature of the Liquid and Dissolved Substance
The type of substance dissolved in the liquid and the chemical nature of the liquid itself affect its conductivity. Strong electrolytes, which completely dissociate into ions, result in higher conductivity than weak electrolytes, which only partially dissociate.
Example: Sulfuric acid, a strong electrolyte, dissociates completely in water and is an excellent conductor. In contrast, acetic acid, a weak electrolyte, only partially dissociates in water, making it a poor conductor of electricity.
4. Type of Ions
The type of ions present in the liquid also influences conductivity. Ions with higher charges or smaller sizes tend to conduct electricity more efficiently. Divalent or multivalent ions (ions with charges of ±2 or more) tend to be more conductive than monovalent ions (ions with a charge of ±1).
Example: Magnesium sulfate (MgSO₄) dissociates into Mg²⁺ and SO₄²⁻ ions in water, which are more conductive than Na⁺ and Cl⁻ ions in sodium chloride solution.
Applications of Electrical Conduction in Liquids
The concept of electrical conduction in liquids has numerous applications across different industries and fields, including electroplating, batteries, electrolysis, and medical treatments. Below are some notable applications that highlight the importance of this phenomenon.
1. Electrolysis
Electrolysis is a process that uses electric current to drive a non-spontaneous chemical reaction. It is commonly used to break down compounds, such as water into hydrogen and oxygen, or to deposit metals onto surfaces in electroplating.
Example: In the electrolysis of water, an electric current is passed through water containing an electrolyte, such as sulfuric acid. Water molecules are broken down into hydrogen and oxygen gases, which can be collected at the electrodes.
![]()
Hydrogen gas is collected at the cathode, while oxygen gas is collected at the anode.
2. Electroplating
Electroplating involves depositing a thin layer of metal onto a surface using an electric current. The object to be plated is placed in a solution containing metal ions, and electric current is applied, causing metal ions to deposit on the object.
Example: In silver electroplating, an object made of a base metal (e.g., copper) is submerged in a silver nitrate solution. When a current is passed through the solution, silver ions (Ag⁺) are reduced at the surface of the object, forming a layer of metallic silver.
![]()
This technique is widely used to enhance the appearance and durability of jewelry, electronics, and automotive parts.
3. Batteries and Electrochemical Cells
Batteries rely on the conduction of electricity through liquids (electrolytes) to generate power. In a typical electrochemical cell, two electrodes are placed in an electrolyte solution, and a chemical reaction between the electrodes generates an electric current.
Example: In a lead-acid battery, commonly used in vehicles, sulfuric acid acts as the electrolyte. The chemical reactions between lead, lead dioxide, and sulfuric acid produce an electric current, powering the vehicle’s starter motor.
4. Saltwater Desalination through Electrodialysis
Electrodialysis is a method of desalinating saltwater by passing an electric current through it to separate salt ions from the water. This process uses ion-selective membranes that allow only certain ions to pass through, effectively reducing the salt concentration in the water.
Example: In electrodialysis, saltwater is placed between alternating cation- and anion-selective membranes. When a voltage is applied, sodium ions move toward the cathode, while chloride ions move toward the anode, producing desalinated water as a by-product.
5. Medical Applications: Electrotherapy
In electrotherapy, a mild electric current is applied to the body through a conductive gel or saline solution. This technique is used in various medical treatments to alleviate pain, stimulate muscles, and improve blood circulation.
Example: Transcutaneous electrical nerve stimulation (TENS) involves placing electrodes on the skin with a conductive gel. The current passed through the gel reduces pain signals by stimulating nerves, offering pain relief in physical therapy.
Challenges and Limitations of Conducting Electricity in Liquids
While conducting electricity in liquids is highly useful, there are certain challenges and limitations associated with this process.
1. Corrosion and Electrolysis: Some electrolytes can cause corrosion of the electrodes over time, especially in electrochemical cells. Electrolysis can lead to the unwanted breakdown of electrolytes and materials.
2. Limited Conductivity: The conductivity of liquid electrolytes is often lower than that of solid metals, making them less efficient for applications requiring high current densities.
3. Temperature Sensitivity: Liquid electrolytes are sensitive to temperature changes. At very high temperatures, liquids may evaporate, while at low temperatures
, they may freeze, affecting their conductivity.
4. Safety and Toxicity: Some electrolytes, such as strong acids or bases, are corrosive and toxic. Their use requires careful handling, containment, and disposal.
Conclusion
The conduction of electricity in liquids is a unique and versatile phenomenon that relies on the movement of ions in electrolyte solutions. By understanding the factors that influence liquid conductivity, such as ion concentration, temperature, and electrolyte type, we can apply this knowledge in numerous practical ways. From essential industrial processes like electrolysis and electroplating to energy storage in batteries and medical treatments, the conduction of electricity in liquids has broad applications that contribute to advancements in technology, healthcare, and environmental sustainability.
Despite its limitations, the conduction of electricity in liquids remains an area of active research and development, particularly as new materials and techniques emerge. Understanding this process continues to offer insights into how we can harness the properties of liquids for efficient energy solutions and diverse scientific applications, making it an indispensable aspect of modern science and engineering.