The dehydration of alcohols is an important organic chemical reaction where water is removed from an alcohol molecule to form an alkene. This process is widely used both in laboratory synthesis and industrial chemistry to produce alkenes, which are key building blocks for many chemical compounds, including plastics, pharmaceuticals, and fuels. The reaction typically requires heat and a catalyst, often an acid, to facilitate the breaking of the alcohol molecule’s hydroxyl group and the formation of a carbon-carbon double bond.

In this article, we will dive deep into the mechanism of alcohol dehydration, explore the conditions under which the reaction occurs, and examine its applications in various industries. We will also look at real-world examples, such as the dehydration of ethanol, and discuss how the process is utilized in the production of important industrial chemicals.
What is Dehydration of Alcohols?
The dehydration of alcohols refers to the chemical reaction in which an alcohol molecule loses a molecule of water (H₂O) to form an alkene. This reaction is typically carried out under acidic conditions, often using catalysts like sulfuric acid (H₂SO₄) or phosphoric acid (H₃PO₄), and requires the application of heat. The dehydration of alcohols is a type of elimination reaction because the reaction involves the elimination of water from the alcohol molecule.
General Reaction Equation
The general reaction for the dehydration of an alcohol is:
![]()
Where:
- R represents the alkyl group attached to the alcohol,
- CH₂OH is the alcohol group (-OH),
- The resulting product is an alkene with a carbon-carbon double bond (CH=CH₂) and a water molecule (H₂O) as a byproduct.
Example: Dehydration of Ethanol
A common example is the dehydration of ethanol (C₂H₅OH) to produce ethylene (C₂H₄). This reaction occurs when ethanol is heated in the presence of an acid catalyst, typically sulfuric acid:
![]()
In this case, ethanol loses a molecule of water and forms ethylene, a key industrial alkene used in the production of polymers like polyethylene.
Mechanism of Dehydration: E1 and E2 Reactions
The dehydration of alcohols can follow two main types of mechanisms: E1 (unimolecular elimination) or E2 (bimolecular elimination). The pathway that the reaction follows depends on the structure of the alcohol and the reaction conditions, particularly the strength of the acid and the temperature.
1. E1 Mechanism (Unimolecular Elimination)
The E1 mechanism is common in the dehydration of tertiary alcohols and some secondary alcohols. It involves a two-step process where the formation of the carbocation is the rate-determining step. The reaction occurs in the following steps:
- Protonation of the Alcohol:
The alcohol is protonated by the acid catalyst, converting the hydroxyl group (-OH) into a better leaving group (H₂O). This protonation makes the -OH group more likely to leave.R-CH₂CH₂OH+H⁺→R-CH₂CH₂OH₂⁺ - Formation of a Carbocation:
Once the hydroxyl group is protonated, it leaves the molecule as a water molecule, creating a carbocation (positively charged carbon atom) in the process. This carbocation is highly reactive.R-CH₂CH₂OH₂⁺→R-CH⁺+H₂O - Elimination of a Proton:
A proton (H⁺) is removed from a neighboring carbon atom, leading to the formation of a double bond between the two carbon atoms. This results in the formation of the alkene.R-CH⁺→-H⁺R-CH=CH₂
The E1 mechanism is favored by tertiary alcohols because the carbocation formed during the reaction is more stable due to the electron-donating nature of the surrounding alkyl groups.
2. E2 Mechanism (Bimolecular Elimination)
The E2 mechanism is more common for the dehydration of primary alcohols and some secondary alcohols. This mechanism involves a one-step process where the elimination of the water molecule and the formation of the carbon-carbon double bond occur simultaneously.
- Protonation of the Alcohol:
Like in the E1 mechanism, the first step is the protonation of the hydroxyl group to make it a better leaving group. - Simultaneous Elimination:
In one concerted step, the water molecule leaves and a proton is removed from a neighboring carbon atom. This results in the simultaneous formation of the alkene and the departure of water.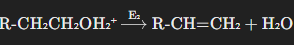
The E2 mechanism is typically favored in primary alcohols because they do not form stable carbocations, so a concerted mechanism without carbocation formation is preferred.
Zaitsev’s Rule in Dehydration
In reactions where multiple alkenes can form, the Zaitsev’s Rule often applies. This rule states that the more substituted alkene (the one with more alkyl groups attached to the double-bonded carbons) will be the major product because it is generally more stable.
For example, in the dehydration of butanol, both 1-butene and 2-butene can form, but according to Zaitsev’s rule, 2-butene (the more substituted alkene) will be the major product.
![]()
Factors Affecting the Dehydration of Alcohols
The rate and efficiency of alcohol dehydration can be influenced by several factors, including:
1. The Structure of the Alcohol
- Tertiary alcohols dehydrate more easily than secondary alcohols, which dehydrate more easily than primary alcohols. This is because the formation of a stable carbocation (which is more likely in tertiary alcohols) speeds up the reaction in E1 mechanisms.
- Primary alcohols typically follow the E2 mechanism, which requires stronger conditions, such as higher temperatures.
2. Temperature
Dehydration reactions are generally endothermic, meaning they require heat to proceed. Higher temperatures favor the elimination of water and the formation of alkenes.
- For primary alcohols, temperatures of 170°C to 180°C are often needed for efficient dehydration.
- Secondary and tertiary alcohols typically dehydrate at lower temperatures (around 100°C to 140°C) due to the stability of the carbocation intermediates.
3. Acid Catalysts
Strong acids such as sulfuric acid (H₂SO₄) or phosphoric acid (H₃PO₄) are commonly used as catalysts to protonate the hydroxyl group of the alcohol. The acid not only provides the proton needed for the reaction but also helps remove the water molecule, driving the equilibrium toward alkene formation.
- Sulfuric acid is commonly used because of its strong dehydrating properties.
- Phosphoric acid is used when a milder acid is preferred, as it reduces the risk of side reactions like oxidation.
Applications of Alcohol Dehydration
The dehydration of alcohols is a useful reaction in both the laboratory and industrial settings. Alkenes produced through dehydration are essential for the manufacture of many chemical products, including plastics, fuels, and solvents.
1. Industrial Production of Ethylene
One of the most important industrial applications of alcohol dehydration is the production of ethylene (C₂H₄) from ethanol. Ethylene is a critical raw material in the chemical industry, used primarily to produce polyethylene, one of the most common plastics. Ethylene is also used to manufacture chemicals such as ethylene oxide and ethylene glycol, which are key components in the production of antifreeze and polyester.
The dehydration of ethanol to ethylene is typically carried out using phosphoric acid on silica as a catalyst at high temperatures:
![]()
This process is highly efficient and widely used in the petrochemical industry.
2. Alkene Synthesis in Organic Chemistry
In organic synthesis, alcohol dehydration is frequently used to produce alkenes, which are versatile intermediates in many chemical reactions. Alkenes can undergo a wide range of reactions, such as hydrohalogenation, hydration, and oxidation, making them valuable starting materials for synthesizing more complex molecules.
Example: Dehydration of 2-Propanol
The dehydration of 2-propanol (isopropanol) produces propene (propylene), a key alkene used in the production of polypropylene, a plastic commonly used in packaging, textiles, and automotive parts.
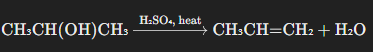
Propene is a critical monomer for the production of polypropylene through polymerization processes.
3. Dehydration in Biofuel Production
The production of bioethanol, which is derived from renewable resources such as sugarcane or corn, involves several dehydration steps. After fermentation produces ethanol, the ethanol can be dehydrated to produce bio-ethylene, which serves as a sustainable alternative to ethylene derived from fossil fuels.
This ethylene can then be used to produce renewable plastics or converted into ethylene glycol for bio-based antifreeze production.
Conclusion
The dehydration of alcohols is a fundamental chemical reaction used to convert alcohols into alkenes through the removal of water. Depending on the structure of the alcohol and the reaction conditions, the process can follow either an E1 or E2 mechanism. Strong acids such as sulfuric or phosphoric acid are typically used as catalysts to promote the elimination of water.
This reaction has numerous applications, from the production of industrial alkenes like ethylene and propene to the synthesis of complex organic molecules. Understanding the dehydration of alcohols is crucial for chemists working in industries ranging from plastics to renewable energy, where alkenes play a pivotal role in the development of new materials and fuels.