The Frenkel defect is a type of point defect in a crystal lattice that occurs when an atom or ion leaves its regular lattice site and occupies an interstitial site within the same crystal structure. Named after Russian physicist Yakov Frenkel, who first proposed it in 1926, this defect plays a significant role in understanding the behavior of materials, especially in ionic crystals. The presence of Frenkel defects can influence the electrical, optical, and thermal properties of materials, making it a key concept in solid-state physics, materials science, and semiconductor engineering.
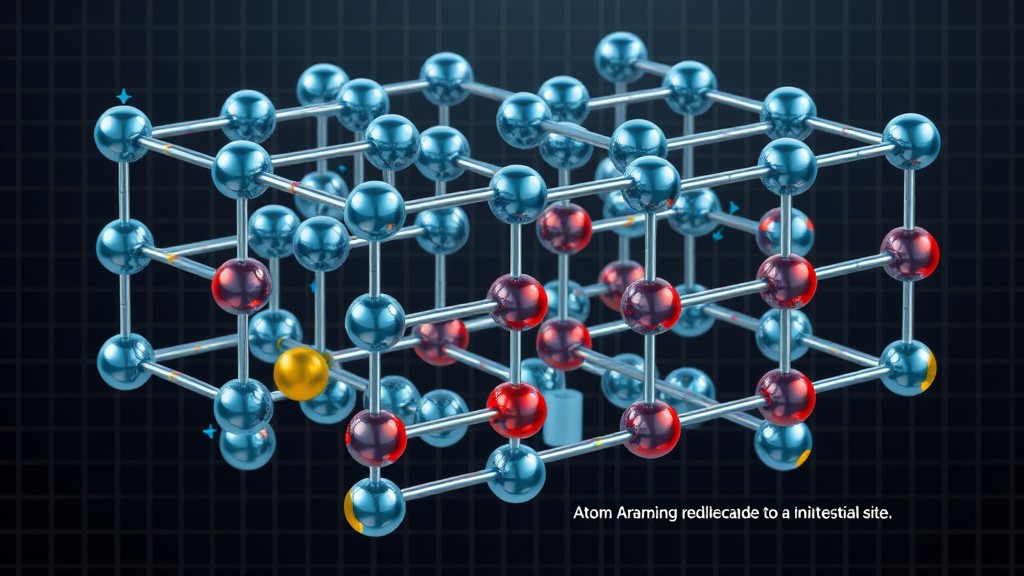
This article explores the concept of Frenkel defects, the conditions under which they form, their effects on material properties, and practical applications in technology.
Understanding Frenkel Defects
In a perfect crystal lattice, atoms or ions are arranged in a highly ordered, repeating pattern. However, real crystals often contain various imperfections or “defects.” The Frenkel defect is one such defect, characterized by the displacement of an ion from its regular lattice site to an interstitial position (a position between regular lattice sites). This leaves behind a vacancy at the original site and creates an interstitial defect at the new location, effectively maintaining the charge balance and chemical composition of the crystal.
Key Characteristics of Frenkel Defects
1. Charge Neutrality: Since the displaced ion remains within the crystal lattice, the overall charge of the lattice remains neutral, and there is no net change in the crystal’s composition.
2. Common in Ionic Crystals: Frenkel defects commonly occur in crystals with ions of significantly different sizes. They are more likely in materials where the anion is much larger than the cation, making it easier for the smaller ion to occupy an interstitial position.
3. Temperature Dependence: The formation of Frenkel defects increases with temperature, as higher thermal energy makes it easier for ions to leave their lattice sites.
Example: In silver chloride (AgCl), a Frenkel defect can occur when a silver ion (Ag![]() ) moves from its regular lattice position to an interstitial site, leaving a vacancy in its original position.
) moves from its regular lattice position to an interstitial site, leaving a vacancy in its original position.
Formation of Frenkel Defects
The formation of Frenkel defects is influenced by several factors, including the crystal structure, ionic sizes, and temperature. In ionic crystals, Frenkel defects are more likely to occur when one ion is significantly smaller than the other, as the smaller ion can more easily occupy interstitial sites without causing significant lattice distortion.
Steps in Frenkel Defect Formation
1. Vacancy Formation: A small ion (usually a cation in ionic crystals) moves from its lattice site, creating a vacancy at its original position.
2. Interstitial Occupation: The ion moves to an interstitial site within the crystal, forming an interstitial defect.
3. Charge Balance Maintenance: Since both the vacancy and the interstitial defect involve the same ion, the total charge in the crystal remains balanced.
Example of Frenkel Defect in Silver Halides
In silver halides, such as silver chloride (AgCl) and silver bromide (AgBr), Frenkel defects occur due to the relatively small size of silver ions (Ag![]() ) compared to the larger halide ions (Cl
) compared to the larger halide ions (Cl![]() and Br
and Br![]() ). At higher temperatures, some silver ions leave their regular lattice positions and occupy interstitial sites, creating Frenkel defects. These defects contribute to the unique electrical and optical properties of silver halides, making them useful in photographic and electronic applications.
). At higher temperatures, some silver ions leave their regular lattice positions and occupy interstitial sites, creating Frenkel defects. These defects contribute to the unique electrical and optical properties of silver halides, making them useful in photographic and electronic applications.
Differences Between Frenkel Defects and Schottky Defects
Frenkel defects and Schottky defects are both point defects in crystals, but they differ in structure and formation.
Frenkel Defects
- Involve a single ion moving from its lattice site to an interstitial position.
- No ions leave the crystal lattice, so there is no net change in mass or density.
- Common in crystals with large differences in ion sizes, such as AgCl and ZnS.
Schottky Defects
- Involve the removal of both cations and anions from the lattice, leaving behind vacancies for both types of ions.
- Reduce the overall density of the crystal due to the loss of mass.
- Common in ionic crystals with similar ion sizes, such as NaCl and KCl.
Example Comparison
In NaCl, a Schottky defect would involve both a sodium ion (Na![]() ) and a chloride ion (Cl
) and a chloride ion (Cl![]() ) leaving the lattice, reducing the crystal’s density. In contrast, in AgCl, a Frenkel defect would involve only the displacement of a silver ion (Ag
) leaving the lattice, reducing the crystal’s density. In contrast, in AgCl, a Frenkel defect would involve only the displacement of a silver ion (Ag![]() ) to an interstitial site, with no change in overall density.
) to an interstitial site, with no change in overall density.
Effects of Frenkel Defects on Material Properties
The presence of Frenkel defects in a crystal lattice can significantly alter the material’s properties, particularly its electrical, optical, and thermal behavior.
1. Electrical Conductivity
Frenkel defects increase the number of vacancies and interstitial ions within a crystal, which can enhance ionic conductivity. In ionic crystals, the presence of these defects allows ions to move more easily through the lattice under an electric field, facilitating ionic conduction.
- Example: In silver bromide (AgBr), Frenkel defects involving silver ions improve ionic conductivity, making AgBr useful in solid-state batteries and other electrochemical devices.
2. Optical Properties
Frenkel defects can alter the optical properties of crystals by affecting light absorption and scattering. In certain crystals, Frenkel defects introduce energy states within the band gap, allowing the material to absorb specific wavelengths of light.
- Example: In silver halides, Frenkel defects are partly responsible for the material’s sensitivity to light, which is exploited in traditional photography, where silver halide crystals form latent images upon exposure to light.
3. Mechanical Properties
Frenkel defects can also affect the mechanical properties of a material. In some cases, these defects introduce points of stress within the lattice, affecting its strength and hardness. The effect on mechanical properties depends on the concentration and distribution of defects within the crystal.
- Example: In certain ceramics, a high concentration of Frenkel defects can weaken the lattice, making it more susceptible to deformation or fracture under stress.
4. Thermal Conductivity
The presence of Frenkel defects can disrupt the orderly arrangement of atoms or ions in the lattice, affecting the transfer of thermal energy through the material. Typically, Frenkel defects reduce thermal conductivity by scattering phonons, the primary carriers of heat in non-metallic solids.
- Example: In ionic crystals like zinc sulfide (ZnS), the presence of Frenkel defects can reduce thermal conductivity, making ZnS potentially useful in applications requiring thermal insulation.
Temperature Dependence and Defect Concentration
The concentration of Frenkel defects in a crystal increases with temperature. As temperature rises, the thermal energy within the lattice increases, making it easier for ions to overcome the potential energy barrier and move to interstitial sites. This temperature dependence follows an Arrhenius-type relationship:
![]()
where:
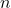 is the defect concentration,
is the defect concentration,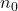 is a constant related to the lattice structure,
is a constant related to the lattice structure, is the formation energy of the defect,
is the formation energy of the defect,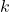 is Boltzmann’s constant,
is Boltzmann’s constant,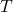 is the absolute temperature.
is the absolute temperature.
As the temperature increases, the exponential factor grows, leading to a higher concentration of Frenkel defects. However, at very high temperatures, excessive defects can destabilize the crystal structure, potentially causing the material to deform or melt.
Applications of Materials with Frenkel Defects
The presence of Frenkel defects makes materials suitable for specific applications where their altered electrical, optical, and thermal properties are advantageous.
1. Solid-State Batteries and Ionic Conductors
Materials with high ionic conductivity due to Frenkel defects are useful in solid-state batteries, where ions must move through a solid electrolyte. The increased conductivity provided by these defects enhances the efficiency and power density of the battery.
- Example: Silver chloride (AgCl) and silver bromide (AgBr) are used in certain types of solid-state batteries due to their high ionic conductivity, which results from Frenkel defects in their lattice.
2. Photographic Materials
Frenkel defects in silver halides, such as silver chloride and silver bromide, increase the material’s sensitivity to light, making these compounds ideal for photographic applications. In traditional photography, light exposure creates a latent image by reducing silver ions, which later develop into visible images.
- Example: In film photography, silver bromide crystals in the film emulsion capture light, forming a latent image that can be developed into a photograph.
3. Semiconductor Devices
Frenkel defects can modify the electrical and optical properties of semiconductors, enabling them to be used in devices where specific conductive or optical properties are required. Certain defects can also act as charge carriers, increasing the material’s conductivity under specific conditions.
- Example: Zinc sulfide (ZnS) is a semiconductor that can have Frenkel defects, making it suitable for use in electroluminescent devices and phosphors in display screens.
4. Thermoelectric Materials
Frenkel defects can reduce thermal conductivity, a desirable property in thermoelectric materials, which convert temperature differences into electrical energy. Lower thermal conductivity helps maintain a temperature gradient across the material, improving thermoelectric efficiency.
- Example: Some ceramic materials exhibit low thermal conductivity due to Frenkel defects, making them useful in thermoelectric devices that require efficient heat-to-electricity conversion.
5. Electrolytes in Fuel Cells
In solid oxide fuel cells (SOFCs), electrolyte materials with Frenkel defects facilitate the transport of ions,
particularly oxygen ions, across the electrolyte. This ionic conductivity is essential for the efficient operation of SOFCs, which rely on ion exchange to generate electricity.
- Example: Materials like zirconium oxide (ZrO
 ) doped with yttrium exhibit ionic conductivity, partly due to defect structures similar to Frenkel defects, making them ideal for SOFC electrolytes.
) doped with yttrium exhibit ionic conductivity, partly due to defect structures similar to Frenkel defects, making them ideal for SOFC electrolytes.
Comparison of Frenkel Defects and Other Defects
Frenkel defects are just one type of point defect, and they have distinct characteristics compared to other defects like Schottky defects and impurity defects.
| Defect Type | Description | Effect on Density | Example Materials |
|---|---|---|---|
| Frenkel Defect | Ion displaces to interstitial site; charge-neutral | No effect on density | AgCl, ZnS |
| Schottky Defect | Equal number of cation and anion vacancies | Decreases density | NaCl, KCl |
| Impurity Defect | Foreign ions or atoms replace lattice ions | Variable effects | Doped semiconductors |
Conclusion
The Frenkel defect is a crucial point defect in crystals, where an ion moves from its regular lattice position to an interstitial site, creating a vacancy and an interstitial defect. This type of defect, common in materials with a significant difference in ion sizes, influences the electrical, optical, thermal, and mechanical properties of materials. The effects of Frenkel defects are widely used in various applications, from solid-state batteries and photographic materials to thermoelectric devices and fuel cells. Understanding Frenkel defects and their behavior enables scientists and engineers to design materials with specific properties tailored to technological needs, advancing innovations in energy storage, electronics, and imaging technologies.