The p-block elements are a significant group of elements in the periodic table, located in groups 13 to 18. These elements have their valence electrons in the p-orbital, which gives them a wide variety of chemical and physical properties. They include some of the most versatile and essential elements, such as carbon, nitrogen, oxygen, and halogens, which are vital for life and numerous industrial applications.
This article introduces the p-block elements, their general properties, and their applications, supported by examples to illustrate their importance in science and everyday life.
What Are p-Block Elements?
The p-block elements are those elements in which the last electron enters the p-orbital of their outermost energy level. These elements belong to groups 13 to 18 of the periodic table and include both metals and non-metals, as well as metalloids. The diversity in their properties arises from the variation in their electronic configurations and the number of valence electrons.
General Characteristics of p-Block Elements
1. Valence Electrons: The p-block elements have 3 to 8 valence electrons. For example:
– Group 13 elements (e.g., boron) have 3 valence electrons.
– Group 18 elements (noble gases) have 8 valence electrons, except helium, which has 2.
2. Electron Configuration: The general electronic configuration of p-block elements is ![]() .
.
3. Variety of States: The p-block elements exhibit solid, liquid, and gaseous states at room temperature:
– Solids: Carbon, phosphorus, and sulfur.
– Liquids: Bromine.
– Gases: Oxygen, nitrogen, and chlorine.
4. Variable Oxidation States: These elements often exhibit multiple oxidation states due to the availability of p-electrons and sometimes d-electrons for bonding.
5. Reactivity: Reactivity varies widely within the p-block:
– Non-metals like fluorine and oxygen are highly reactive.
– Noble gases are inert due to their full valence shell.
6. Diversity: p-Block elements include non-metals, metalloids, and metals, making this group highly versatile.
Groups in the p-Block
The p-block spans six groups in the periodic table, each with unique characteristics:
1. Group 13 (Boron Group): Boron, aluminum, gallium, indium, and thallium.
2. Group 14 (Carbon Group): Carbon, silicon, germanium, tin, and lead.
3. Group 15 (Nitrogen Group): Nitrogen, phosphorus, arsenic, antimony, and bismuth.
4. Group 16 (Oxygen Group): Oxygen, sulfur, selenium, tellurium, and polonium.
5. Group 17 (Halogens): Fluorine, chlorine, bromine, iodine, and astatine.
6. Group 18 (Noble Gases): Helium, neon, argon, krypton, xenon, and radon.
Properties of p-Block Elements
1. Metals, Non-Metals, and Metalloids
The p-block is the only block in the periodic table that contains all three types of elements:
- Non-Metals: Carbon, nitrogen, oxygen, and halogens are typical non-metals, characterized by high electronegativity and ionization energy.
- Metalloids: Elements like boron, silicon, and arsenic show properties intermediate between metals and non-metals.
- Metals: Aluminum, tin, and lead are examples of metals in the p-block.
Example: Silicon, a metalloid, is used in semiconductors because of its intermediate electrical conductivity, which can be modified by doping.
2. Chemical Reactivity
The chemical reactivity of p-block elements varies across groups:
- Group 13: Reactivity increases down the group. Aluminum reacts with oxygen to form a protective oxide layer.
- Group 14: Carbon forms covalent compounds, while lead shows metallic properties.
- Group 15: Nitrogen is stable due to its triple bond, but phosphorus reacts readily with oxygen.
- Group 16: Oxygen is highly reactive, while sulfur forms compounds like sulfides.
- Group 17: Halogens are extremely reactive, especially fluorine, which reacts with almost all elements.
- Group 18: Noble gases are mostly inert, with xenon forming compounds under extreme conditions.
Example: Fluorine reacts with hydrogen to form hydrogen fluoride (![]() ), a highly reactive and corrosive compound.
), a highly reactive and corrosive compound.
3. Oxidation States
p-Block elements exhibit a variety of oxidation states, often depending on the number of valence electrons and their ability to participate in bonding:
- Group 13: +3 and +1 (e.g., aluminum forms
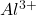 ).
). - Group 14: +4 and +2 (e.g., carbon forms
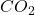 in the +4 state and
in the +4 state and 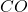 in the +2 state).
in the +2 state). - Group 15: +5, +3, and -3 (e.g., nitrogen forms
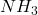 in the -3 state and
in the -3 state and 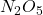 in the +5 state).
in the +5 state). - Group 16: -2, +4, and +6 (e.g., sulfur forms
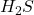 in the -2 state and
in the -2 state and 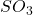 in the +6 state).
in the +6 state). - Group 17: -1 (e.g., chlorine forms
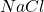 ).
). - Group 18: Rare oxidation states, except xenon, which can form compounds like
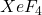 .
.
4. Allotropes
Some p-block elements exhibit allotropy, where they exist in different structural forms:
- Carbon: Graphite, diamond, and fullerenes.
- Phosphorus: White phosphorus, red phosphorus, and black phosphorus.
- Oxygen:
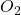 (dioxygen) and
(dioxygen) and 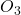 (ozone).
(ozone).
Example: Graphite conducts electricity due to delocalized electrons, while diamond is an insulator with a rigid tetrahedral structure.
Applications of p-Block Elements
1. Group 13: Boron and Aluminum
- Boron: Used in detergents, glass, and ceramics. Boron compounds like boric acid are antiseptics.
- Aluminum: Widely used in construction, transportation, and packaging due to its lightweight and corrosion resistance.
Example: Aluminum foil is used to wrap food because it is non-toxic and prevents contamination.
2. Group 14: Carbon and Silicon
- Carbon: Forms the basis of organic compounds and fuels. It is used in diamonds for jewelry and graphite for lubricants.
- Silicon: Essential for semiconductors, solar panels, and glass production.
Example: Silicon chips are used in computers and smartphones, enabling modern technology.
3. Group 15: Nitrogen and Phosphorus
- Nitrogen: Found in fertilizers, explosives, and as a coolant in its liquid form.
- Phosphorus: Used in fertilizers, detergents, and safety matches.
Example: Ammonia (![]() ), derived from nitrogen, is a key component of agricultural fertilizers.
), derived from nitrogen, is a key component of agricultural fertilizers.
4. Group 16: Oxygen and Sulfur
- Oxygen: Vital for respiration and used in welding and medical applications.
- Sulfur: Used in vulcanizing rubber, producing sulfuric acid, and as a fungicide.
Example: Sulfuric acid is a crucial industrial chemical used in battery production and fertilizer manufacturing.
5. Group 17: Halogens
- Fluorine: Used in toothpaste (as fluoride) and in the production of Teflon.
- Chlorine: Used to disinfect water and in the production of PVC (polyvinyl chloride).
- Iodine: Essential for thyroid health and used as an antiseptic.
Example: Chlorine is added to swimming pools to kill harmful bacteria.
6. Group 18: Noble Gases
- Helium: Used in balloons and as a coolant in cryogenics.
- Neon: Used in advertising signs and high-voltage indicators.
- Argon: Used as an inert gas in welding and in light bulbs.
- Xenon: Used in high-intensity lamps and as an anesthetic.
Example: Argon gas is used in incandescent light bulbs to prevent the tungsten filament from oxidizing.
Conclusion
The p-block elements are among the most versatile and essential elements in the periodic table, influencing life, industry, and technology. From the life-sustaining properties of oxygen and nitrogen to the technological innovations enabled by silicon and aluminum, these elements form the backbone of countless applications. Understanding their properties, behaviors, and uses provides a foundation for advancements in science, engineering, and sustainability. As our knowledge of these elements expands, so too will their impact on society and the environment.