Manganese (Mn) is a transition metal and a vital element with diverse applications in industry, biology, and environmental science. Known for its strength and versatility, manganese is primarily used in steel production, but it also plays an essential role in biochemistry as a trace mineral crucial to various enzymatic processes in living organisms. With its distinct characteristics and valuable properties, manganese is a critical element in both natural and industrial processes.

In this article, we’ll explore manganese’s atomic structure, physical and chemical properties, occurrence in nature, industrial applications, and biological significance, providing a comprehensive understanding of this remarkable element.
Atomic Structure and Properties of Manganese
Manganese is a transition metal with atomic number 25 and is represented by the symbol Mn on the periodic table. It belongs to the d-block of the periodic table and has a partially filled d-orbital, making it one of the elements with variable oxidation states and complex chemical behavior.
Atomic Structure and Electron Configuration
- Atomic Number: 25
- Atomic Symbol: Mn
- Atomic Weight: 54.938 g/mol
- Electron Configuration: [Ar]
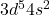
- Oxidation States: +2, +3, +4, +6, +7 (with +2 and +7 being the most stable)
The electron configuration of manganese reveals that it has five electrons in its 3d subshell and two in the 4s subshell. The partially filled d-orbitals contribute to manganese’s ability to exhibit multiple oxidation states, which is essential to its versatility in chemical reactions and applications.
Physical Properties of Manganese
Manganese has distinct physical properties that make it suitable for various industrial applications:
- Appearance: Manganese is a hard, brittle metal with a silvery-gray color. It tarnishes when exposed to air and can oxidize rapidly.
- Density: 7.21 g/cm³
- Melting Point: 1246 °C (2275 °F)
- Boiling Point: 2061 °C (3742 °F)
- Hardness: Manganese is relatively hard but brittle, making it difficult to work with in its pure form.
These properties make manganese useful as an alloying agent, as it can significantly enhance the strength, hardness, and resistance of other metals without adding much weight.
Chemical Properties of Manganese
Manganese exhibits a variety of chemical properties due to its variable oxidation states, which range from +2 to +7. These oxidation states make manganese highly versatile in redox reactions, where it can act as both an oxidizing and a reducing agent.
- Reactivity: Manganese is moderately reactive, oxidizing when exposed to air to form manganese oxides. It reacts with dilute acids, releasing hydrogen gas.
- Oxides: Manganese forms several oxides, including MnO, MnO₂, Mn₂O₃, and MnO₄⁻. Each oxide has different properties and uses, particularly MnO₂, which is a powerful oxidizing agent.
- Complex Compounds: Manganese readily forms complex compounds and can act as a catalyst in various chemical reactions.
Example: In the reaction between manganese dioxide (MnO₂) and hydrogen peroxide (H₂O₂), MnO₂ acts as a catalyst to decompose H₂O₂ into water and oxygen gas. This reaction is commonly demonstrated in laboratories to showcase catalytic behavior:
![]()
Occurrence and Extraction of Manganese
Manganese is the twelfth most abundant element in the Earth’s crust and is primarily found in oxide ores. It is never found in its pure elemental form in nature due to its reactivity; instead, it commonly occurs as manganese dioxide (MnO₂) and other manganese oxides.
Major Manganese Ores
The main ores of manganese include:
- Pyrolusite (MnO₂): The most important ore of manganese, pyrolusite is a black mineral that is widely mined for manganese production.
- Rhodochrosite (MnCO₃): This manganese carbonate mineral is commonly found with other manganese oxides.
- Manganite (Mn₂O₃·H₂O): A hydrated oxide of manganese.
Extraction Process
Manganese is primarily extracted from pyrolusite through reduction processes. The process involves heating manganese dioxide with carbon or aluminum to reduce it to metallic manganese:
1. Reduction with Carbon: Pyrolusite is heated with carbon in a blast furnace, resulting in the reduction of MnO₂ to manganese metal.
![]()
2. Reduction with Aluminum (Thermite Process): In cases where high-purity manganese is required, aluminum is used as the reducing agent. The thermite process produces high-quality manganese metal.
Industrial Applications of Manganese
Manganese is crucial in several industries, particularly in steel production and metallurgy, due to its ability to improve the properties of other metals. Below are some significant applications of manganese:
1. Steel Production and Alloys
Manganese is essential in the production of steel, where it acts as a deoxidizer and alloying agent. It improves the strength, hardness, and durability of steel while also enhancing its resistance to wear and deformation. Manganese is commonly used in various steel types, including high-strength steels and stainless steels.
- Example: In the production of high-carbon steel, manganese reduces the brittleness of the alloy by removing impurities like sulfur and oxygen. This creates a steel that is more durable and resistant to cracking.
2. Batteries and Electronics
Manganese dioxide (MnO₂) is widely used in dry cell batteries, including alkaline batteries, where it acts as a cathode material. The high oxidizing ability of MnO₂ makes it effective in converting chemical energy into electrical energy, making manganese-based batteries reliable and long-lasting.
- Example: Alkaline batteries use manganese dioxide as the positive electrode (cathode) material. MnO₂ reacts with zinc to produce electric current, which powers devices like flashlights, remote controls, and electronic toys.
3. Chemical Industry
Manganese compounds, particularly manganese dioxide, are used in various chemical reactions and industrial processes as catalysts and oxidizing agents. MnO₂ is also used in water treatment to remove iron and other impurities.
- Example: In the glass industry, manganese dioxide is used to remove color impurities caused by iron in glass production. MnO₂ acts as a bleaching agent, turning the glass colorless or slightly purple to counteract the green hue caused by iron.
4. Agriculture
Manganese is an essential micronutrient for plant growth and is often added to fertilizers. It is required for photosynthesis and the formation of chlorophyll, which is essential for plant development. Manganese fertilizers help improve soil health and increase crop yields.
- Example: Manganese sulfate (
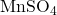 ) is a common additive in fertilizers to supplement soil manganese levels, especially in soils deficient in this micronutrient.
) is a common additive in fertilizers to supplement soil manganese levels, especially in soils deficient in this micronutrient.
5. Pigments and Ceramics
Manganese compounds, especially manganese oxides, are used as pigments in ceramics and paints. These compounds produce rich brown, purple, and black colors, making them suitable for pottery and other decorative arts.
- Example: Manganese dioxide is used to create brown and black glazes in ceramics, giving pottery its distinctive color and enhancing its aesthetic appeal.
Biological Importance of Manganese
Manganese is a trace mineral essential to all forms of life. In the human body, it plays a role in various enzymatic processes and is vital for bone health, metabolism, and antioxidant defenses.
Role in Enzyme Activation
Manganese is a cofactor for several enzymes that are crucial in metabolic processes. It activates enzymes involved in amino acid, carbohydrate, and cholesterol metabolism, allowing the body to process these essential nutrients.
- Example: Manganese is a cofactor for superoxide dismutase (MnSOD), an enzyme that protects cells from oxidative damage by breaking down superoxide radicals. This function is critical in maintaining cellular health and preventing oxidative stress.
Contribution to Bone Health
Manganese is essential for bone formation and health, as it helps in the synthesis of bone matrix proteins. Alongside other minerals like calcium and magnesium, manganese contributes to bone density and structural integrity.
- Example: Manganese deficiency in humans can lead to bone weakness and skeletal abnormalities, as manganese is necessary for bone mineralization and collagen production.
Antioxidant Properties
Manganese-containing enzymes help protect cells from oxidative stress by neutralizing free radicals. This antioxidant effect reduces the risk of cellular damage, inflammation, and degenerative diseases.
- Example: The antioxidant enzyme superoxide dismutase (MnSOD) in mitochondria reduces oxidative stress, lowering the risk of age-related conditions such as arthritis, cardiovascular disease, and neurodegeneration.
Manganese Deficiency and Toxicity
While manganese is essential in small amounts, both deficiency and excess manganese can have negative effects on health.
- Deficiency: Manganese deficiency is rare but can lead to bone problems, impaired growth, and metabolism-related disorders. Symptoms of deficiency include joint pain, fatigue, and poor wound healing
.
- Toxicity: Excessive manganese exposure, usually through industrial work or environmental pollution, can lead to manganese toxicity, affecting the nervous system and potentially causing symptoms like tremors, memory problems, and cognitive impairment.
Environmental Impact of Manganese
While manganese is naturally abundant and essential, excessive manganese in water and soil due to industrial pollution can have detrimental environmental effects. High concentrations of manganese can affect aquatic ecosystems and plant health.
Example: Manganese in Drinking Water
Excess manganese in drinking water can lead to discoloration and health concerns. Manganese can stain plumbing fixtures and affect water quality, and prolonged exposure can impact neurological health.
Manganese in Soil and Agriculture
Soil with high levels of manganese may inhibit the growth of some plants. Conversely, soils deficient in manganese can lead to crop deficiencies, affecting agricultural productivity and crop quality.
Conclusion
Manganese is a versatile and essential element with unique chemical and physical properties that make it valuable across various industries. Its role in steel production, battery manufacturing, agriculture, and biochemistry highlights its wide range of applications and importance. As a trace mineral, manganese supports crucial biological functions, including enzyme activation, bone health, and antioxidant protection. Despite its numerous benefits, responsible management is necessary to avoid environmental pollution and toxicity. Overall, manganese’s presence in both industrial applications and biological systems underlines its significance as a critical element in the natural world and human advancement.