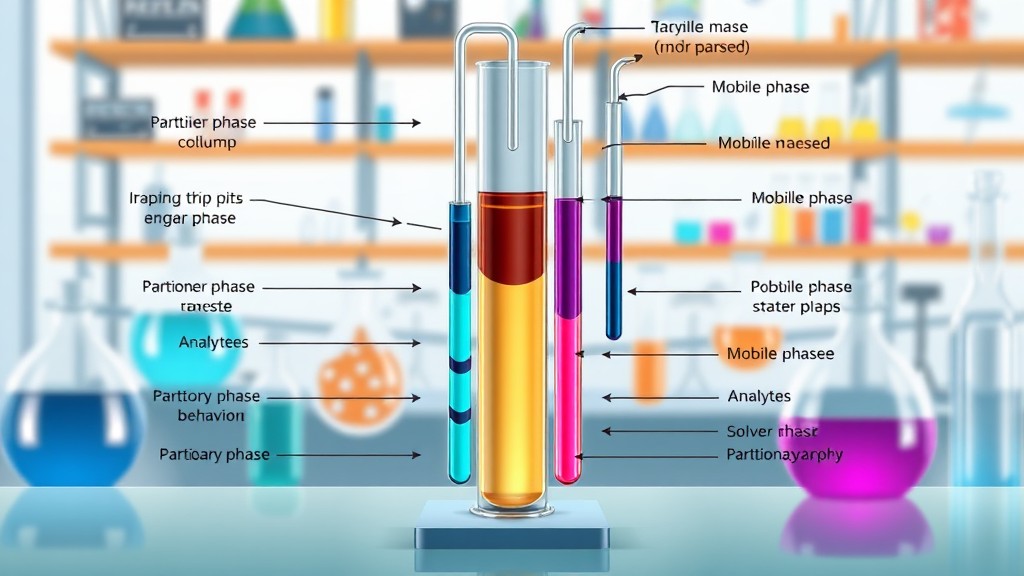
Partition chromatography is a separation technique used to isolate and analyze components in a mixture based on their differences in solubility between two immiscible liquid phases. Developed as an essential tool in chemistry and biochemistry, partition chromatography relies on the partitioning of solutes between a stationary phase and a mobile phase. The principle is similar to liquid-liquid extraction but with a continuous process that allows for efficient separation and analysis.
This article provides an in-depth overview of partition chromatography, including its working principle, types, methods, and applications, with examples to demonstrate its utility in various fields.
Understanding the Principles of Partition Chromatography
Partition chromatography operates on the concept of partition coefficient or distribution coefficient (![]() ), which describes how a compound distributes itself between two immiscible phases. In partition chromatography, these two phases are typically liquids:
), which describes how a compound distributes itself between two immiscible phases. In partition chromatography, these two phases are typically liquids:
- The stationary phase is a liquid that is either adsorbed onto a solid support or bound within the matrix of a stationary material.
- The mobile phase is a liquid that moves through the stationary phase, carrying the components of the sample with it.
Partition Coefficient and Separation
The partition coefficient ![]() is a ratio that quantifies how a compound is distributed between the two phases at equilibrium:
is a ratio that quantifies how a compound is distributed between the two phases at equilibrium:
![]()
A compound with a higher affinity for the stationary phase will have a larger ![]() and will spend more time in the stationary phase, moving slowly. Conversely, compounds with a greater affinity for the mobile phase will have a lower
and will spend more time in the stationary phase, moving slowly. Conversely, compounds with a greater affinity for the mobile phase will have a lower ![]() and will move more quickly through the system.
and will move more quickly through the system.
Mechanism of Partitioning
As the mobile phase passes through the stationary phase, each component of the mixture partitions, or distributes itself, according to its solubility and affinity for each phase. This continuous partitioning process allows components to separate as they move through the column at different rates based on their partition coefficients.
Example: Consider a mixture of two compounds, A and B, where compound A has a higher affinity for the stationary phase than compound B. As the mobile phase flows through the stationary phase, compound B moves more quickly, eluting from the system before compound A, leading to a separation of the two.
Types of Partition Chromatography
Partition chromatography can be classified into different types based on the nature of the stationary and mobile phases, the support material, and the mode of operation.
1. Liquid-Liquid Partition Chromatography
In liquid-liquid partition chromatography, both the stationary and mobile phases are liquids. The stationary phase is typically coated onto an inert solid support, and the mobile phase is an immiscible liquid that flows through the stationary phase. This setup allows the solutes to partition between two liquid phases continuously.
- Example: Paper chromatography is a form of liquid-liquid partition chromatography, where water is often used as the stationary phase (absorbed by the paper fibers), and an organic solvent acts as the mobile phase.
2. Gas-Liquid Partition Chromatography (GLPC)
In gas-liquid partition chromatography, the mobile phase is a gas (usually an inert gas like helium or nitrogen), while the stationary phase is a liquid film coated onto a solid support within a column. This type is commonly used for separating volatile compounds and is especially useful in analytical applications like gas chromatography (GC).
- Example: Gas chromatography with a polar stationary phase can separate a mixture of hydrocarbons based on differences in volatility and polarity, allowing identification and quantification of each component.
3. Thin-Layer Chromatography (TLC)
Thin-layer chromatography is a type of partition chromatography where the stationary phase is a thin layer of liquid adsorbed on a solid surface, such as silica gel or alumina, coated on a glass or plastic plate. The mobile phase is typically an organic solvent that moves up the plate by capillary action, separating the components based on partitioning.
- Example: In TLC, a mixture of organic dyes can be separated based on their partition coefficients between the stationary phase (silica gel) and the mobile phase (an organic solvent like ethanol), with each dye traveling a unique distance on the TLC plate.
4. High-Performance Liquid Chromatography (HPLC)
High-performance liquid chromatography, a sophisticated type of partition chromatography, uses high pressure to force the mobile phase through a densely packed column with a stationary phase. HPLC enables high-resolution separation of complex mixtures, making it one of the most effective analytical techniques for both qualitative and quantitative analysis.
- Example: In pharmaceutical analysis, HPLC can separate active ingredients in a drug formulation, allowing precise measurement of each component’s concentration.
Methods in Partition Chromatography
There are several key methods in partition chromatography, each suitable for different sample types and analytical goals. The two most common methods are column chromatography and planar chromatography.
Column Chromatography
Column chromatography is performed in a vertical column packed with a stationary phase. The mobile phase, carrying the sample, is applied at the top of the column and flows downward due to gravity or pressure. As the mobile phase moves through the column, components in the sample partition between the two phases and separate based on their partition coefficients.
- Example: In biochemistry, column chromatography is often used to purify proteins, with specific stationary and mobile phases chosen to optimize separation based on protein solubility and charge.
Planar Chromatography
In planar chromatography, such as paper or thin-layer chromatography, the stationary phase is spread out on a flat surface (paper or glass plate). The mobile phase moves across the surface by capillary action or solvent front, separating components as it moves. This method is especially popular in laboratories for quick, preliminary analysis.
- Example: Paper chromatography can separate pigments in plant extracts, where different pigments travel different distances depending on their solubility in the mobile phase and affinity for the paper.
Applications of Partition Chromatography
Partition chromatography has a wide range of applications across industries, including pharmaceuticals, environmental science, food analysis, and biochemistry. Its ability to separate complex mixtures with precision makes it a powerful tool in both research and industrial processes.
1. Pharmaceutical Analysis
Partition chromatography, especially HPLC, is widely used in the pharmaceutical industry for drug analysis and quality control. It enables precise separation and quantification of active ingredients, impurities, and degradation products in pharmaceutical formulations.
- Example: In drug manufacturing, HPLC is used to ensure that each tablet contains the correct dosage of active ingredients and meets purity standards by separating and analyzing all chemical components.
2. Environmental Analysis
Partition chromatography is essential in environmental science for detecting and quantifying pollutants, pesticides, and contaminants in water, soil, and air samples. By separating components based on their partition coefficients, scientists can identify and measure the concentration of hazardous substances in the environment.
- Example: Gas-liquid partition chromatography is used to analyze pesticide residues in soil, separating various pesticide compounds and measuring their concentrations to assess environmental impact.
3. Food and Beverage Industry
Partition chromatography is applied in food and beverage quality control to detect food additives, flavor compounds, and potential contaminants. It also helps in verifying authenticity and origin by analyzing the chemical composition of food products.
- Example: Liquid-liquid partition chromatography is used to separate and identify flavor compounds in essential oils, allowing quality control in the production of flavors and fragrances.
4. Biochemical Analysis
In biochemistry, partition chromatography is crucial for separating biomolecules such as amino acids, proteins, and nucleotides. It helps researchers purify and analyze these molecules, which is essential for studying cellular processes, enzyme functions, and genetic material.
- Example: Partition chromatography can be used to separate different amino acids in a protein hydrolysate. By comparing the separated components to known standards, biochemists can identify the amino acids present in a sample.
5. Forensic Science
Forensic laboratories use partition chromatography to analyze complex biological and chemical samples from crime scenes. This analysis is essential for identifying substances such as drugs, toxins, and organic compounds that can serve as evidence in criminal investigations.
- Example: Gas chromatography-mass spectrometry (GC-MS) is used to identify traces of drugs or toxins in biological samples. The partition chromatography component (GC) separates compounds, while the mass spectrometer detects and identifies each component with high accuracy.
Advantages and Limitations of Partition Chromatography
Advantages
1. High Resolution: Partition chromatography provides high-resolution separation, enabling the identification of individual components in complex mixtures.
2. Versatility: It can be applied to a wide variety of substances, including small molecules, biomolecules, and volatile compounds.
3. Quantitative and Qualitative Analysis: It allows both quantitative and qualitative analysis, providing precise measurements and structural information.
Limitations
1. Limited to Certain Phases: Partition chromatography is generally limited to samples that can be dissolved in liquid or gas phases, making it less suitable for solid materials.
2. Time and Cost: Certain types, like HPLC, can be time-consuming and require expensive equipment.
3. Sensitivity to Conditions: Partitioning efficiency is sensitive to conditions such as temperature, solvent composition, and pressure, requiring careful optimization for accurate results.
Practical Example of Partition Chromatography: Separation of Plant Pigments
One classic example of partition chromatography is the separation of plant pigments using paper chromatography. A mixture of pigments, such as chlorophyll, carotene, and xanthophylls, is applied to a paper strip and developed using a nonpolar solvent as the mobile phase.
Steps in the Procedure
1. A spot of plant extract is placed on the paper strip near the base.
2. The paper is placed in a container with
a solvent that gradually travels up the paper by capillary action.
3. As the solvent moves, each pigment partitions between the stationary water phase in the paper fibers and the mobile organic phase.
4. Different pigments travel different distances based on their partition coefficients.
The result is a colorful separation of pigments, with each pigment forming a distinct spot on the paper. Chlorophyll (green), carotene (orange), and xanthophyll (yellow) are visually separated, allowing for easy identification and analysis of plant pigments.
Conclusion
Partition chromatography is a versatile and powerful technique for separating complex mixtures based on the differences in partitioning behavior between two immiscible phases. With its wide applicability in pharmaceuticals, environmental science, food safety, biochemistry, and forensics, partition chromatography has become indispensable for both research and industrial applications. By understanding the principles, types, and applications of partition chromatography, scientists and engineers can develop innovative methods to analyze and purify compounds, ensuring quality and safety across various fields. Whether in basic research or practical applications, partition chromatography remains a foundational tool in modern analytical chemistry.