Benzene (C₆H₆) is one of the most well-known aromatic hydrocarbons in organic chemistry. It holds a unique place in the study of chemistry due to its distinctive structure, which has led to much research and curiosity over the years. Benzene is a simple compound with a molecular formula of C₆H₆, consisting of six carbon atoms and six hydrogen atoms, arranged in a ring. However, the actual structure of benzene is far more complex and interesting than it first appears.

This article explores the structure of benzene in detail, covering its historical discovery, its resonance hybrid structure, molecular geometry, bonding, and physical and chemical properties. By examining the characteristics of benzene, we can understand why it plays a critical role in organic chemistry, particularly in the formation of aromatic compounds.
Historical Discovery of Benzene
The structure of benzene puzzled scientists for decades after its discovery due to its unsaturated chemical formula, which suggests that it should be highly reactive, like other alkenes (hydrocarbons with double bonds). However, benzene exhibits much more stability than expected. This discrepancy led to years of study to unravel its structure.
Benzene was first discovered in 1825 by the English scientist Michael Faraday, who isolated it from illuminating gas. Later, in 1865, Friedrich August Kekulé, a German chemist, proposed a groundbreaking idea that benzene was a ring of carbon atoms. Kekulé famously stated that he envisioned the ring structure of benzene in a dream, where he saw a snake biting its own tail, representing the cyclical nature of the carbon atoms in benzene.
This hexagonal ring model laid the foundation for the modern understanding of benzene’s structure, although further research revealed additional complexities that extended beyond Kekulé’s original suggestion.
Structure of Benzene
Benzene’s molecular formula, C₆H₆, means it consists of six carbon atoms and six hydrogen atoms. Despite this simple molecular formula, benzene’s stability and its lack of typical alkene reactivity led scientists to explore a more detailed structural explanation.
1. Kekulé Structure
Kekulé’s structure for benzene was based on alternating single and double bonds between the carbon atoms in a hexagonal ring. This can be represented as follows:
H H | | H-C=C-C=C-H | | H-C=C-C-H | | H H
This alternating bond structure is sometimes called the Kekulé model, where three carbon-carbon bonds are single bonds, and the other three are double bonds. However, this structure presented a problem: if benzene had distinct alternating single and double bonds, it would be expected to exhibit the reactivity of alkenes, where double bonds undergo addition reactions. In reality, benzene is much more stable than typical alkenes, and it does not readily participate in addition reactions.
2. Resonance Structure of Benzene
The stability of benzene cannot be fully explained by Kekulé’s alternating single and double bond model. Instead, it is now understood that benzene exists as a resonance hybrid. Resonance occurs when a molecule can be represented by two or more equivalent structures that differ only in the placement of electrons. In benzene, the electrons in the double bonds are delocalized, meaning they are spread out evenly across all six carbon atoms in the ring, rather than being confined to specific carbon-carbon bonds.
In resonance terms, benzene is often depicted as a hybrid of two structures, represented by alternating single and double bonds:
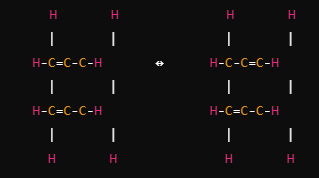
However, the actual structure of benzene is a blend of these resonance structures. The delocalization of electrons means that all the carbon-carbon bonds in the ring are equivalent, and each bond has a bond order of 1.5 (rather than 1 for a single bond or 2 for a double bond). The delocalized electrons form a continuous π-electron cloud above and below the plane of the ring, contributing to the molecule’s unusual stability.
3. Aromaticity and Stability of Benzene
Benzene’s extraordinary stability is attributed to its aromaticity. A compound is considered aromatic if it follows the Hückel rule, which states that a cyclic, planar molecule is aromatic if it has 4n+2 π-electrons (where nn is a whole number). Benzene fits this criterion because it has six π-electrons (from the three double bonds), and when n=1, the Hückel rule gives 4(1)+2=6 π-electrons.
This delocalization of electrons over the ring creates a stabilizing effect that lowers the overall energy of the molecule, making benzene much less reactive than would be expected for a compound with alternating double bonds. Benzene resists reactions that would disrupt its aromaticity, such as addition reactions, and instead tends to undergo substitution reactions, where one substituent is replaced by another, allowing the aromatic ring to remain intact.
4. Bond Length in Benzene
One of the most significant pieces of evidence supporting the resonance model of benzene is the bond length between the carbon atoms. In typical alkenes, single carbon-carbon bonds are longer (about 1.54 Å) than double bonds (about 1.34 Å). In benzene, however, all the carbon-carbon bond lengths are identical and have a length of about 1.39 Å, which is between that of a single and double bond. This uniform bond length supports the idea of delocalized electrons and a resonance hybrid structure.
5. Molecular Geometry of Benzene
Benzene has a planar (flat) hexagonal structure, where all carbon atoms are arranged in a perfect hexagon, and all bond angles between the carbon atoms are 120 degrees. This planar structure is important for maintaining the delocalization of the π-electrons, which is crucial for benzene’s aromaticity.
Each carbon atom in the benzene ring is sp² hybridized, meaning that each carbon forms three sigma (σ) bonds—two with neighboring carbon atoms and one with a hydrogen atom. The unhybridized p-orbitals of the carbon atoms overlap to form the delocalized π-system above and below the plane of the ring.
6. Representation of Benzene
To simplify the structure of benzene, chemists often use a hexagonal ring with a circle inside it to represent the delocalized π-electrons. This representation emphasizes the equivalence of all the carbon-carbon bonds and the resonance nature of the molecule.
_______ / \ | | \_______/
The circle in the middle of the hexagon symbolizes the delocalized electrons, rather than showing alternating single and double bonds. This is a widely accepted representation of aromatic compounds like benzene.
Chemical Properties of Benzene
The unique structure of benzene gives rise to several interesting chemical properties, most notably its stability and resistance to typical reactions of alkenes. Here, we will examine how benzene’s structure influences its chemical behavior.
1. Resistance to Addition Reactions
Alkenes, which contain carbon-carbon double bonds, typically undergo addition reactions, where new atoms are added to the carbons of the double bond. However, benzene does not readily participate in addition reactions because doing so would disrupt its aromaticity.
For example, while alkenes react with bromine (Br2) to form dibromo compounds, benzene resists this reaction. Instead, benzene typically undergoes electrophilic aromatic substitution reactions, where a hydrogen atom in the ring is replaced by another substituent, such as a halogen or nitro group, but the aromatic ring remains intact.
2. Electrophilic Aromatic Substitution (EAS)
Benzene’s most common reaction type is electrophilic aromatic substitution (EAS). In these reactions, an electrophile (a species that seeks electrons) reacts with the π-electron cloud of benzene. However, rather than breaking the ring’s structure, one of the hydrogen atoms attached to a carbon atom is substituted by the electrophile.
Example: The bromination of benzene is a typical electrophilic aromatic substitution reaction. In the presence of a catalyst, such as ferric bromide (FeBr3), benzene reacts with bromine to form bromobenzene (C6H5Br):
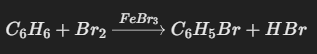
3. Stability and Heat of Hydrogenation
The heat of hydrogenation is a measure of the energy released when hydrogen is added to a compound to convert double bonds into single bonds. If benzene had three distinct double bonds, its heat of hydrogenation would be much higher than what is observed. In reality, benzene’s heat of hydrogenation is much lower because of the stability provided by its aromatic ring. This further supports the idea of delocalized electrons and resonance stabilization in benzene.
4. Reactivity with Substituents
When substituents are attached to the benzene ring, they can affect its reactivity. For example, electron-donating groups (such as hydroxyl or alkyl groups) tend to make benzene more reactive toward electrophiles, while electron-withdrawing groups (such as nitro groups) make the ring less reactive.
The position of the substituents on the benzene ring also plays a role in determining the outcome of substitution reactions. Some groups direct new substituents to the ortho and para positions, while others direct them to the meta position, influencing the structure and properties of the resulting compound.
Uses and Importance of Benzene
Benzene’s structure and properties make it a fundamental building block in the production of a wide variety of chemical products. Although benzene itself is a toxic and carcinogenic substance, it is used as a precursor for the synthesis of many important compounds.
- Production of Plastics: Benzene is used in the production of styrene, which is polymerized to make polystyrene, a plastic material used in packaging, insulation, and disposable containers.
- Pharmaceuticals: Many drugs are synthesized from benzene derivatives. For example, aspirin is derived from salicylic acid, which contains a benzene ring.
- Dyes and Synthetic Fibers: Benzene is used in the manufacture of aniline, a precursor to various dyes and synthetic fibers like nylon.
- Rubber: Benzene is a key raw material in the production of synthetic rubber, which is used in tires and various industrial applications.
Conclusion
The structure of benzene is one of the most important and interesting concepts in organic chemistry. The delocalization of electrons in its resonance hybrid structure provides benzene with unusual stability, making it the foundation of aromatic chemistry. Understanding the bonding, geometry, and reactivity of benzene allows chemists to predict its behavior in various reactions and utilize it as a key intermediate in the production of numerous chemical products.
From its role in the manufacture of plastics and pharmaceuticals to its impact on the study of aromaticity, benzene’s structure continues to fascinate and challenge chemists, remaining one of the most significant discoveries in the world of chemistry.