One of the most distinctive and crucial properties of carbon is its tetravalency. This characteristic makes carbon the backbone of countless organic molecules, allowing it to form stable compounds essential for life. Tetravalency refers to carbon’s ability to form four covalent bonds, a result of having four valence electrons. These four bonds enable carbon to create a vast array of molecules, including chains, rings, and complex structures like DNA and proteins.
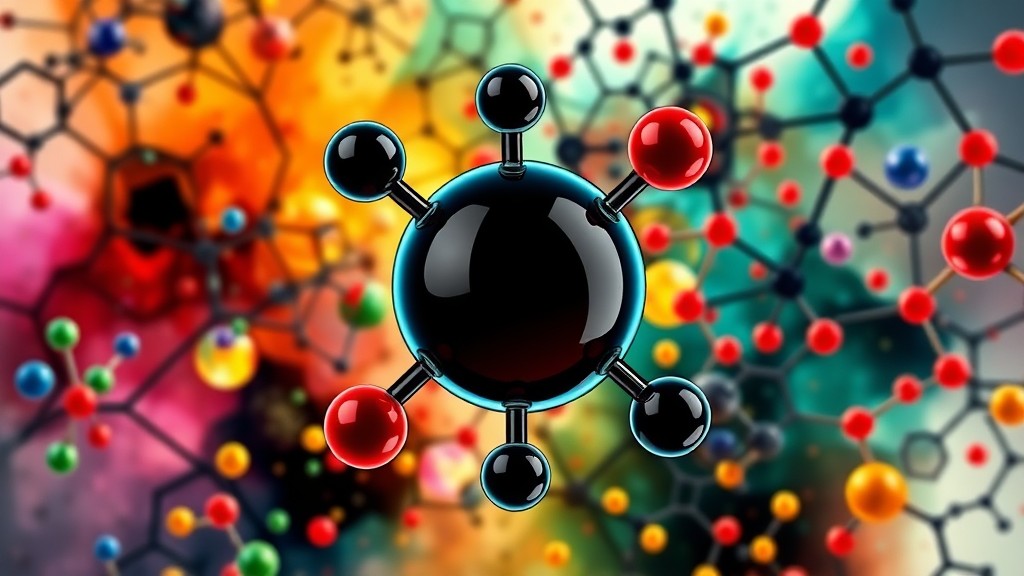
This article delves into the tetravalency of carbon, explaining its basis in atomic structure, how it enables complex molecular formation, and examples of its impact in organic chemistry and biological processes.
Understanding Tetravalency in Carbon
Carbon has an atomic number of 6, with an electron configuration of ![]() . This means it has four valence electrons in its outermost shell (two in the
. This means it has four valence electrons in its outermost shell (two in the ![]() orbital and two in the
orbital and two in the ![]() orbitals). Since a stable electron configuration requires eight electrons in the outer shell, carbon has the capacity to form four covalent bonds with other atoms to satisfy the octet rule.
orbitals). Since a stable electron configuration requires eight electrons in the outer shell, carbon has the capacity to form four covalent bonds with other atoms to satisfy the octet rule.
Electronic Configuration of Carbon
The electron configuration of carbon is as follows:
![]()
In this configuration:
- The 1s orbital is fully occupied by two core electrons.
- The outer shell (valence shell) consists of two electrons in the
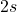 orbital and two electrons in the
orbital and two electrons in the 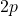 orbital.
orbital.
This configuration gives carbon four valence electrons, which it shares with other atoms to form stable molecules.
Why Does Carbon Form Four Bonds?
To reach a stable octet, carbon needs four additional electrons. It achieves this by forming four covalent bonds through electron sharing. Unlike elements that tend to lose or gain electrons, carbon shares electrons to avoid the high energy cost associated with forming ions. As a result, carbon forms covalent bonds, establishing its tetravalency and allowing it to bond with a variety of elements, including hydrogen, oxygen, nitrogen, and other carbon atoms.
Example: Methane (CH₄) – A Simple Tetravalent Structure
Methane (CH₄) is a straightforward example of carbon’s tetravalency:
- In methane, carbon forms four single covalent bonds with four hydrogen atoms.
- Each hydrogen atom contributes one electron, while carbon shares its four valence electrons with the hydrogens.
The molecular structure of methane is tetrahedral, with bond angles of approximately 109.5°, illustrating how carbon’s tetravalency enables stable and symmetrical bonding.
Importance of Tetravalency in Organic Chemistry
The tetravalency of carbon is central to organic chemistry, where carbon serves as the backbone of organic compounds. Carbon’s ability to form four bonds allows it to build stable structures of varying shapes, lengths, and complexities, from simple molecules like methane to vast macromolecules like proteins and DNA. The versatility of carbon’s tetravalency supports the formation of functional groups, aromatic rings, and multiple bond types.
Structural Diversity Due to Tetravalency
1. Chain Structures: Carbon atoms can link together to form chains of varying lengths, either as straight chains or branched chains. This is seen in alkanes (like propane and butane), where carbon forms single bonds with itself and other atoms.
2. Ring Structures: Carbon’s tetravalency also allows it to form ring structures, as in cycloalkanes and aromatic compounds like benzene. These rings are stable and provide the basis for numerous organic molecules.
3. Multiple Bonds: Carbon’s tetravalency allows for double bonds (as in alkenes) and triple bonds (as in alkynes), leading to varied bonding types and reactivities. For example, ethylene (C₂H₄) has a double bond between the carbon atoms, while acetylene (C₂H₂) has a triple bond.
Example: Ethylene (C₂H₄) – A Molecule with a Double Bond
In ethylene:
- Each carbon atom forms two single bonds with hydrogen atoms and a double bond with the other carbon atom.
- This satisfies carbon’s tetravalency, with each carbon having a total of four bonds.
The double bond introduces additional stability and rigidity to the structure, which is essential for the function of many organic molecules.
Types of Covalent Bonds Formed Due to Tetravalency
Carbon’s tetravalency allows it to form different types of covalent bonds, depending on how it shares electrons with other atoms.
1. Single Bonds (Sigma Bonds)
A single bond (sigma bond) forms when two atoms share a pair of electrons. Single bonds are the most basic and common type of bond formed by carbon, providing stability and flexibility to the molecule.
- Example: In ethane (C₂H₆), each carbon atom forms a single bond with the other carbon and three single bonds with hydrogen atoms.
2. Double Bonds (One Sigma and One Pi Bond)
A double bond consists of one sigma bond and one pi bond, allowing for a more rigid and reactive structure than a single bond.
- Example: In ethylene (C₂H₄), a double bond between the two carbon atoms limits the rotation around the bond, which affects the molecule’s shape and reactivity.
3. Triple Bonds (One Sigma and Two Pi Bonds)
A triple bond has one sigma bond and two pi bonds, creating a very strong, linear bond.
- Example: In acetylene (C₂H₂), a triple bond between the two carbons results in a linear molecule, where each carbon forms a single bond with a hydrogen atom.
The combination of these bond types allows carbon to form an extensive range of structures, contributing to its central role in chemistry.
Hybridization in Carbon Compounds
The concept of hybridization helps explain the bonding structure and geometry of carbon compounds. Through hybridization, carbon’s ![]() and
and ![]() orbitals mix to form new hybrid orbitals, accommodating four bonds in different geometrical arrangements.
orbitals mix to form new hybrid orbitals, accommodating four bonds in different geometrical arrangements.
Types of Hybridization in Carbon
1. sp³ Hybridization: In sp³ hybridization, one ![]() orbital and three
orbital and three ![]() orbitals combine to form four sp³ hybrid orbitals, each containing a single electron. This arrangement leads to a tetrahedral geometry with bond angles of 109.5°.
orbitals combine to form four sp³ hybrid orbitals, each containing a single electron. This arrangement leads to a tetrahedral geometry with bond angles of 109.5°.
– Example: Methane (CH₄) has sp³ hybridized orbitals, resulting in a tetrahedral shape.
2. sp² Hybridization: In sp² hybridization, one ![]() orbital and two
orbital and two ![]() orbitals combine to form three sp² hybrid orbitals and one unhybridized
orbitals combine to form three sp² hybrid orbitals and one unhybridized ![]() orbital. This results in a trigonal planar geometry with bond angles of 120°.
orbital. This results in a trigonal planar geometry with bond angles of 120°.
– Example: Ethylene (C₂H₄) has sp² hybridized orbitals, with a double bond between the two carbons and a planar geometry.
3. sp Hybridization: In sp hybridization, one ![]() orbital and one
orbital and one ![]() orbital combine to form two sp hybrid orbitals, resulting in a linear geometry with bond angles of 180°.
orbital combine to form two sp hybrid orbitals, resulting in a linear geometry with bond angles of 180°.
– Example: Acetylene (C₂H₂) has sp hybridized orbitals, with a triple bond between the carbons and a linear structure.
The concept of hybridization is fundamental in explaining the shapes and angles of carbon compounds, as well as their stability and reactivity.
Importance of Tetravalency in Biological Molecules
Carbon’s tetravalency is a crucial feature in the formation of biological macromolecules, such as carbohydrates, lipids, proteins, and nucleic acids. The ability of carbon to form stable, complex structures with diverse functional groups allows it to be the backbone of life.
1. Carbohydrates
In carbohydrates like glucose (C₆H₁₂O₆), carbon forms chains and rings with hydroxyl groups (–OH) attached, giving the molecule its unique structure and properties. The versatility of carbon bonding enables carbohydrates to serve as an essential energy source in living organisms.
2. Lipids
Lipids, which include fats and oils, are composed primarily of long chains of carbon and hydrogen. Carbon’s tetravalency allows these long chains to form stable, non-polar structures that are vital for cell membranes and energy storage.
3. Proteins
Proteins consist of amino acids, each containing a central carbon atom bonded to an amino group (–NH₂), a carboxyl group (–COOH), a hydrogen atom, and a variable R-group. The central carbon atom, known as the alpha carbon, enables the diverse structures of proteins due to its four bonding sites, leading to complex 3D configurations.
4. Nucleic Acids
In DNA and RNA, the carbon atoms in the sugar-phosphate backbone form stable covalent bonds, allowing for the double-helix structure and encoding of genetic information. The tetravalency of carbon in nucleic acids enables the stability and functionality
of genetic material.
Applications of Carbon’s Tetravalency
The tetravalency of carbon has wide-ranging applications in both nature and technology. Its ability to form stable, diverse structures makes it invaluable in areas such as material science, energy storage, and pharmaceuticals.
1. Material Science: Polymers and Plastics
Polymers, such as polyethylene and polypropylene, are long chains of carbon-based molecules. The versatility of carbon bonding allows these polymers to be used in various applications, from packaging materials to high-strength composites used in construction.
2. Pharmaceuticals
Carbon’s tetravalency enables the design of complex drug molecules that interact precisely with biological targets. Many pharmaceuticals contain carbon chains or rings with functional groups, allowing for specific interactions with enzymes or receptors in the body.
3. Energy Storage: Hydrocarbons and Batteries
Carbon-based hydrocarbons, like methane, propane, and gasoline, are primary energy sources due to their high energy content. Additionally, in batteries, carbon materials like graphite serve as electrode materials due to their stability and conductivity.
4. Nanotechnology: Carbon Nanotubes and Graphene
Carbon’s tetravalency has enabled the development of advanced materials like graphene and carbon nanotubes. These materials exhibit remarkable strength, flexibility, and conductivity, with applications in electronics, medicine, and environmental technology.
Conclusion: The Impact of Carbon’s Tetravalency
The tetravalency of carbon is foundational to the diversity and complexity of organic chemistry. By forming four covalent bonds, carbon creates stable, versatile structures that are essential for life and countless technological applications. From simple molecules like methane to complex biomolecules and advanced materials, carbon’s ability to connect with various atoms underpins the molecular diversity in chemistry, making it a unique and indispensable element. The impact of carbon’s tetravalency extends beyond biology to fields such as material science, medicine, and nanotechnology, highlighting its central role in both natural and engineered systems.