The mole concept is one of the cornerstones of chemistry, providing a bridge between the atomic world and the macroscopic world of grams, liters, and other measurable quantities. A “mole” is a standard scientific unit used to measure large numbers of tiny entities like atoms, molecules, ions, or electrons. This concept simplifies calculations in chemistry, allowing scientists to quantify and balance chemical reactions with ease.

The mole concept was formalized to deal with the vast numbers involved in atomic-scale measurements, enabling chemists to connect experimental data with theoretical models. This article explains the definition of a mole, its relationship to Avogadro’s number, and its application in chemical reactions, along with detailed examples.
Definition of a Mole
A mole is defined as the amount of substance containing exactly ![]() elementary entities, such as atoms, molecules, or ions. This number is called Avogadro’s number and is a fundamental constant in chemistry.
elementary entities, such as atoms, molecules, or ions. This number is called Avogadro’s number and is a fundamental constant in chemistry.
What Does a Mole Represent?
The mole concept is similar to other counting units:
- A dozen refers to 12 items (e.g., 12 eggs).
- A mole refers to
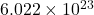 entities (e.g.,
entities (e.g., 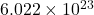 atoms of hydrogen).
atoms of hydrogen).
Because individual atoms and molecules are extraordinarily small, a mole provides a practical way to quantify these entities in terms of measurable amounts like grams or liters.
Example: A Mole of Water Molecules
One mole of water molecules (![]() ) contains
) contains ![]() water molecules. To put this in perspective, this is approximately the number of grains of sand on all the beaches of Earth.
water molecules. To put this in perspective, this is approximately the number of grains of sand on all the beaches of Earth.
Avogadro’s Number and Molar Mass
Avogadro’s Number ( )
)
Avogadro’s number, ![]() , defines how many particles are in one mole of a substance. This constant allows scientists to convert between the atomic scale (particles, atoms, or molecules) and macroscopic quantities (grams or liters).
, defines how many particles are in one mole of a substance. This constant allows scientists to convert between the atomic scale (particles, atoms, or molecules) and macroscopic quantities (grams or liters).
Molar Mass
The molar mass of a substance is the mass of one mole of its particles, expressed in grams per mole (![]() ). The molar mass is numerically equal to the atomic or molecular mass in atomic mass units (
). The molar mass is numerically equal to the atomic or molecular mass in atomic mass units (![]() ).
).
Example: Molar Mass of Water (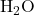 )
)
- Hydrogen (
 ): Atomic mass = 1.008 u
): Atomic mass = 1.008 u - Oxygen (
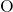 ): Atomic mass = 16.00 u
): Atomic mass = 16.00 u - Molar mass of water =
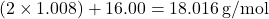
This means 1 mole of water molecules weighs approximately 18.016 grams.
Using the Mole Concept in Calculations
1. Converting Moles to Particles
To find the number of particles in a given number of moles, multiply by Avogadro’s number:
![]()
Example:
How many molecules are in 0.5 moles of oxygen gas (![]() )?
)?
![]()
2. Converting Particles to Moles
To determine the number of moles from a given number of particles, divide by Avogadro’s number:
![]()
Example:
How many moles are in ![]() molecules of carbon dioxide (
molecules of carbon dioxide (![]() )?
)?
![]()
3. Relating Mass, Moles, and Molar Mass
The relationship between mass, moles, and molar mass is given by:
![]()
Example:
How many moles are in 36 grams of water?
- Molar mass of water =
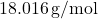
- Mass =

![]()
4. Volume and the Mole Concept (Gases)
At standard temperature and pressure (STP), 1 mole of any ideal gas occupies a volume of ![]() .
.
Example:
What volume does 3 moles of nitrogen gas (![]() ) occupy at STP?
) occupy at STP?
![]()
Application of the Mole Concept in Chemical Reactions
The mole concept simplifies stoichiometric calculations, allowing chemists to determine the proportions of reactants and products in a chemical reaction.
Example: Combustion of Methane (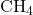 )
)
The balanced equation for methane combustion is:
![]()
This equation shows:
- 1 mole of methane reacts with 2 moles of oxygen to produce 1 mole of carbon dioxide and 2 moles of water.
Problem:
If 5 moles of ![]() are burned, how many moles of
are burned, how many moles of ![]() are produced?
are produced?
- From the balanced equation: 1 mole
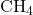 produces 1 mole
produces 1 mole 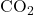 .
. - For 5 moles
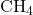 :
:
![]()
Mass Calculation:
To find the mass of ![]() produced, multiply by its molar mass (
produced, multiply by its molar mass (![]() ):
):
![]()
Real-World Examples of the Mole Concept
1. Pharmaceuticals
The mole concept helps in determining the precise amounts of active ingredients in medicines. For instance, knowing the molar mass of a drug allows pharmacists to calculate dosages accurately.
2. Environmental Science
The mole concept is essential in quantifying pollutants, such as calculating the amount of ![]() emitted by burning fossil fuels.
emitted by burning fossil fuels.
Example:
Burning 1 mole of octane (![]() ) releases 8 moles of
) releases 8 moles of ![]() . This relationship helps estimate the environmental impact of vehicle emissions.
. This relationship helps estimate the environmental impact of vehicle emissions.
3. Food Chemistry
Food labels often mention molecular weights of nutrients like proteins and sugars. Using the mole concept, scientists determine how molecules like glucose (![]() ) metabolize to produce energy.
) metabolize to produce energy.
Limitations of the Mole Concept
While the mole concept is invaluable, it has limitations:
1. Assumption of Ideal Behavior: For gases, the mole-volume relationship (![]() ) is accurate only under ideal conditions (STP).
) is accurate only under ideal conditions (STP).
2. Complex Mixtures: In mixtures, identifying the number of moles for individual components can be challenging without additional data.
Conclusion
The mole concept is a fundamental tool in chemistry that connects the microscopic world of atoms and molecules to the macroscopic quantities we can measure. By enabling precise calculations of mass, volume, and particle numbers, the mole simplifies the analysis of chemical reactions, stoichiometry, and real-world applications. Whether determining the dosage of a drug or the amount of carbon dioxide released in a reaction, the mole concept remains a cornerstone of chemical science.