Organic reactions form the foundation of organic chemistry, describing the ways in which organic compounds undergo chemical changes. These reactions are essential to understanding biochemical processes, drug synthesis, materials science, and countless industrial applications. Organic reactions are generally categorized based on the type of chemical transformation involved, such as substitution, addition, elimination, and rearrangement reactions.

This article explores the primary types of organic reactions, providing definitions, mechanisms, and examples to illustrate each category. Understanding these reactions allows us to predict and manipulate the behavior of organic molecules in various chemical and biological contexts.
1. Substitution Reactions
In a substitution reaction, one atom or group of atoms in a molecule is replaced by another atom or group. Substitution reactions are common in organic chemistry and are especially important for reactions involving halogens, alcohols, and amines. They can be classified into two main types: nucleophilic substitution and electrophilic substitution.
A. Nucleophilic Substitution
Nucleophilic substitution reactions involve a nucleophile (a species with a pair of electrons that it can donate) attacking an electron-deficient center, typically a carbon atom attached to a leaving group. There are two main types of nucleophilic substitution reactions:
1. ![]() Reaction (Unimolecular Nucleophilic Substitution): This reaction proceeds in two steps, starting with the departure of the leaving group to form a carbocation intermediate. The nucleophile then attacks the carbocation, resulting in the substitution product.
Reaction (Unimolecular Nucleophilic Substitution): This reaction proceeds in two steps, starting with the departure of the leaving group to form a carbocation intermediate. The nucleophile then attacks the carbocation, resulting in the substitution product. ![]() reactions are common with tertiary alkyl halides.
reactions are common with tertiary alkyl halides.
Example: The reaction of tert-butyl chloride (![]() ) with water follows an
) with water follows an ![]() mechanism, where water replaces the chlorine atom.
mechanism, where water replaces the chlorine atom.
2. ![]() Reaction (Bimolecular Nucleophilic Substitution): In an
Reaction (Bimolecular Nucleophilic Substitution): In an ![]() reaction, the nucleophile attacks the carbon attached to the leaving group in a single, concerted step, resulting in the replacement of the leaving group. This reaction is common in primary and secondary alkyl halides.
reaction, the nucleophile attacks the carbon attached to the leaving group in a single, concerted step, resulting in the replacement of the leaving group. This reaction is common in primary and secondary alkyl halides.
Example: The reaction between methyl bromide (![]() ) and hydroxide ion (
) and hydroxide ion (![]() ) is an
) is an ![]() reaction, where the hydroxide ion replaces the bromine atom.
reaction, where the hydroxide ion replaces the bromine atom.
B. Electrophilic Substitution
Electrophilic substitution reactions involve an electrophile (a species that seeks electrons) replacing a functional group, typically in aromatic compounds. These reactions are important in modifying benzene rings and other aromatic systems.
Example: The nitration of benzene is an electrophilic substitution reaction where a nitronium ion (![]() ) replaces one of the hydrogen atoms on the benzene ring, forming nitrobenzene. This reaction requires concentrated sulfuric and nitric acids.
) replaces one of the hydrogen atoms on the benzene ring, forming nitrobenzene. This reaction requires concentrated sulfuric and nitric acids.
2. Addition Reactions
Addition reactions occur when two or more atoms or groups add to a molecule, typically an unsaturated compound with double or triple bonds. These reactions are commonly seen in alkenes and alkynes, as the double or triple bonds provide sites for addition. Addition reactions can be classified based on the type of attacking reagent: electrophilic, nucleophilic, or free radical.
A. Electrophilic Addition
In electrophilic addition reactions, an electrophile attacks the electron-rich double or triple bond, leading to the addition of new groups to the molecule. This reaction is common in alkenes and alkynes.
Example: The addition of hydrogen bromide (![]() ) to ethene (
) to ethene (![]() ) is an electrophilic addition reaction. The
) is an electrophilic addition reaction. The ![]() from
from ![]() first attaches to one of the carbons, forming a carbocation intermediate. The bromide ion (
first attaches to one of the carbons, forming a carbocation intermediate. The bromide ion (![]() ) then attaches to the carbocation, yielding bromoethane (
) then attaches to the carbocation, yielding bromoethane (![]() ).
).
B. Nucleophilic Addition
Nucleophilic addition reactions occur when a nucleophile attacks a carbonyl group (carbon-oxygen double bond) in an aldehyde or ketone. This type of addition is essential in the synthesis of alcohols, carboxylic acids, and other organic compounds.
Example: In the addition of hydrogen cyanide (![]() ) to acetone (
) to acetone (![]() ), the nucleophilic cyanide ion (
), the nucleophilic cyanide ion (![]() ) attacks the carbonyl carbon, breaking the double bond. The addition yields a cyanohydrin, which has both hydroxyl and nitrile groups attached to the same carbon.
) attacks the carbonyl carbon, breaking the double bond. The addition yields a cyanohydrin, which has both hydroxyl and nitrile groups attached to the same carbon.
C. Free Radical Addition
Free radical addition involves radicals, highly reactive species with unpaired electrons. This type of addition is common in polymerization reactions, where monomers with double bonds join together to form polymers.
Example: The addition of ethylene (![]() ) monomers in the presence of a radical initiator leads to the formation of polyethylene, a widely used plastic material.
) monomers in the presence of a radical initiator leads to the formation of polyethylene, a widely used plastic material.
3. Elimination Reactions
Elimination reactions involve the removal of atoms or groups from a molecule, typically resulting in the formation of double or triple bonds. These reactions are essentially the opposite of addition reactions and are classified based on the mechanism as either ![]() or
or ![]() elimination.
elimination.
A. 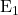 Elimination
Elimination
In an ![]() (unimolecular elimination) reaction, the leaving group first departs, forming a carbocation intermediate. This is followed by the removal of a proton, leading to the formation of a double bond.
(unimolecular elimination) reaction, the leaving group first departs, forming a carbocation intermediate. This is followed by the removal of a proton, leading to the formation of a double bond. ![]() reactions are common with tertiary substrates.
reactions are common with tertiary substrates.
Example: The dehydration of tert-butyl alcohol (![]() ) in the presence of sulfuric acid follows an
) in the presence of sulfuric acid follows an ![]() mechanism. The hydroxyl group leaves, forming a carbocation, and a proton is lost to produce isobutene (
mechanism. The hydroxyl group leaves, forming a carbocation, and a proton is lost to produce isobutene (![]() ).
).
B. 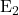 Elimination
Elimination
In ![]() (bimolecular elimination) reactions, the base removes a proton from the substrate at the same time that the leaving group departs. This concerted mechanism results in the formation of a double bond and is common in secondary and primary substrates.
(bimolecular elimination) reactions, the base removes a proton from the substrate at the same time that the leaving group departs. This concerted mechanism results in the formation of a double bond and is common in secondary and primary substrates.
Example: In the dehydrohalogenation of ethyl bromide (![]() ) with a strong base, such as sodium ethoxide, the base removes a hydrogen atom while the bromine atom leaves, resulting in ethylene (
) with a strong base, such as sodium ethoxide, the base removes a hydrogen atom while the bromine atom leaves, resulting in ethylene (![]() ).
).
4. Rearrangement Reactions
Rearrangement reactions involve the reorganization of atoms or groups within a molecule to form a new structure. These reactions often proceed through intermediate carbocations or radicals and result in the formation of more stable isomers. Rearrangement reactions are essential in synthetic organic chemistry for creating complex molecules.
Example: The pinacol rearrangement occurs when pinacol (2,3-dimethyl-2,3-butanediol) is treated with an acid, leading to a rearrangement that forms pinacolone (3,3-dimethyl-2-butanone). The hydroxyl groups are removed, and a carbocation is rearranged to stabilize the molecule.
5. Oxidation and Reduction (Redox) Reactions
Oxidation and reduction reactions, commonly called redox reactions, involve the transfer of electrons. In organic chemistry, these reactions often involve changes in the bonding of carbon to hydrogen or oxygen, where oxidation refers to the loss of electrons (or increase in oxygen bonds), and reduction refers to the gain of electrons (or increase in hydrogen bonds).
A. Oxidation
Oxidation reactions in organic chemistry often involve the conversion of alcohols to aldehydes, ketones, or carboxylic acids. Oxidation increases the number of bonds to oxygen or other electronegative atoms.
Example: The oxidation of ethanol (![]() ) with potassium dichromate (
) with potassium dichromate (![]() ) yields acetaldehyde (
) yields acetaldehyde (![]() ) and then acetic acid (
) and then acetic acid (![]() ) if oxidation continues.
) if oxidation continues.
B. Reduction
Reduction reactions involve the addition of hydrogen or removal of oxygen. Reduction reactions are often used to convert aldehydes and ketones to alcohols or to
reduce double and triple bonds in hydrocarbons.
Example: The reduction of butanone (![]() ) with sodium borohydride (
) with sodium borohydride (![]() ) results in the formation of 2-butanol, where the carbonyl group is converted to a hydroxyl group.
) results in the formation of 2-butanol, where the carbonyl group is converted to a hydroxyl group.
6. Condensation Reactions
Condensation reactions involve the combination of two molecules to form a larger molecule with the elimination of a small molecule, such as water or methanol. These reactions are important in the synthesis of complex molecules, especially in polymer chemistry and biochemistry.
Example: The esterification reaction between an alcohol and a carboxylic acid forms an ester and water. When acetic acid (![]() ) and ethanol (
) and ethanol (![]() ) react, they form ethyl acetate (
) react, they form ethyl acetate (![]() ) and water.
) and water.
Applications of Organic Reactions
The various types of organic reactions are crucial for numerous real-world applications, ranging from pharmaceuticals to materials science and biochemistry.
1. Pharmaceuticals: Synthesis of drugs often involves substitution, addition, and redox reactions to create specific molecular structures with desired biological effects.
2. Polymer Manufacturing: Addition and condensation reactions are widely used to synthesize polymers, including plastics, nylons, and polyester.
3. Food Industry: Oxidation reactions are significant in the preservation of food and the study of antioxidants, as they influence food spoilage and stability.
4. Biochemistry: Rearrangement and redox reactions are key in metabolic pathways, where enzymes catalyze complex reactions essential to life.
Conclusion
Understanding the different types of organic reactions—substitution, addition, elimination, rearrangement, oxidation-reduction, and condensation—is essential in both academic and applied chemistry. Each type of reaction follows distinct mechanisms and contributes to the synthesis and transformation of organic compounds. From drug synthesis to food chemistry, the principles of organic reactions underpin critical processes that impact our daily lives and support technological advancements. With knowledge of organic reactions, chemists can design pathways to create new molecules, materials, and solutions across diverse fields.