Pressure is a fundamental concept in physics and engineering that describes the force applied over a given area. Whether it’s the pressure of the air we breathe, the pressure exerted by water in pipes, or the pressure inside a car tire, understanding how pressure works is essential to many fields, including fluid mechanics, thermodynamics, and even weather forecasting.

In this article, we will explore what pressure is, the units used to measure it, and how pressure plays a role in various practical applications. We will delve into the key units of pressure, including the pascal, atmosphere, bar, and torr, providing examples to illustrate their real-world significance.
What is Pressure?
Pressure is defined as the amount of force exerted per unit area on the surface of an object. Mathematically, pressure is expressed by the equation:
![]()
where:
- P represents pressure,
- F represents the force applied (measured in newtons, N),
- A represents the area over which the force is distributed (measured in square meters, m²).
Pressure can occur in various contexts, from a solid object pressing down on a surface to the force exerted by a gas or liquid on the walls of a container. In gases and liquids, pressure is the result of molecules moving and colliding with the surfaces they encounter.
Example: Tire Pressure
A practical example of pressure is the air pressure inside a car tire. The air molecules inside the tire are constantly moving and colliding with the tire walls, exerting a force. This force per unit area creates what we measure as tire pressure. Proper tire pressure is essential for vehicle performance, safety, and fuel efficiency.
If the force increases (by adding more air), or if the surface area decreases (if the tire volume shrinks), the pressure inside the tire will increase. Conversely, if air leaks from the tire, the pressure decreases because there are fewer molecules exerting force on the walls of the tire.
Units of Pressure
Several units are used to measure pressure, depending on the application or industry. The SI (International System of Units) unit of pressure is the pascal (Pa), but other units such as bar, atmosphere (atm), torr, and pounds per square inch (psi) are also widely used in various contexts.
1. Pascal (Pa)
The pascal (Pa) is the SI unit of pressure, named after the French mathematician and physicist Blaise Pascal. One pascal is defined as one newton of force applied over an area of one square meter:
1Pa=1N/m2
Although the pascal is the standard unit, it is relatively small for most practical applications. For this reason, pressure is often measured in kilopascals (kPa), which equals 1,000 pascals.
- 1 kPa = 1,000 Pa
Example: Atmospheric Pressure
At sea level, the average atmospheric pressure is approximately 101.3 kPa. This is the pressure exerted by the weight of the Earth’s atmosphere on the surface of the planet. It is an essential factor in weather patterns, altitude calculations, and even human respiration.
2. Atmosphere (atm)
The atmosphere (atm) is another common unit of pressure, especially used in meteorology, aviation, and diving. One atmosphere is defined as the average pressure exerted by the Earth’s atmosphere at sea level:
1 atm=101,325 Pa
The atmosphere unit provides an easy reference for air pressure since the average sea-level pressure is close to 1 atm. The atmosphere unit is particularly useful in understanding environmental pressures or when dealing with changes in altitude.
Example: Diving and Atmospheres
As a diver descends underwater, the pressure increases by approximately 1 atm for every 10 meters of depth due to the weight of the water above. At 10 meters below the surface, a diver experiences 2 atmospheres of pressure: 1 atm from the atmosphere above the water and 1 atm from the water itself. This pressure affects the gases the diver breathes, making understanding pressure crucial for diving safety.
3. Bar
The bar is a metric unit of pressure that is widely used in industries such as meteorology and hydrology. One bar is defined as 100,000 pascals, slightly less than one atmosphere.
1 bar=100,000 Pa=0.987 atm
Although the bar is not part of the SI system, it is still commonly used because it offers a convenient unit size for pressures encountered in everyday applications. For example, the bar is frequently used to describe pressures in weather forecasting and automotive tire pressures.
Example: Weather Systems
In meteorology, atmospheric pressure is often measured in hectopascals (hPa) or millibars (mbar), where:
- 1 hPa = 100 Pa
- 1 mbar = 1 hPa
In weather forecasting, high and low-pressure systems are measured in millibars. A typical pressure reading in weather reports might be 1013 mbar, which is equivalent to standard atmospheric pressure at sea level.
4. Torr
The torr is a unit of pressure named after the Italian scientist Evangelista Torricelli, who invented the barometer. The torr is used primarily in fields such as vacuum technology and is closely related to the millimeter of mercury (mmHg):
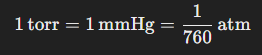
This unit is often used when measuring pressure in low-pressure systems, such as in laboratories or when creating vacuums.
Example: Medical Blood Pressure
In medicine, blood pressure is typically measured in millimeters of mercury (mmHg). A normal blood pressure reading is around 120/80 mmHg, where 120 mmHg represents the pressure when the heart beats (systolic pressure), and 80 mmHg represents the pressure when the heart is at rest (diastolic pressure).
5. Pounds per Square Inch (psi)
In the United States, the unit pounds per square inch (psi) is commonly used to measure pressure. It is especially popular in automotive, aviation, and industrial applications.
1 psi=6894.76 Pa
Psi measures the amount of force in pounds applied to an area of one square inch.
Example: Tire Pressure in Vehicles
In the automotive industry, tire pressure is commonly measured in psi. A typical car tire might be inflated to a pressure of 30 to 35 psi, which ensures optimal performance and safety. Low tire pressure can cause increased friction with the road, reducing fuel efficiency and increasing wear on the tire, while excessively high pressure can lead to reduced traction and a harsher ride.
Applications of Pressure in Everyday Life
Pressure is a critical concept that affects a wide range of everyday activities, from the functioning of our internal body systems to the operation of complex machines.
1. Blood Pressure in the Human Body
Blood pressure is one of the most important vital signs for assessing cardiovascular health. It measures the pressure exerted by circulating blood on the walls of blood vessels, particularly arteries. Maintaining normal blood pressure is essential for ensuring that oxygen and nutrients are adequately delivered to the body’s tissues.
Example: Hypertension (high blood pressure) is a medical condition where the pressure in the arteries is consistently elevated, increasing the risk of heart disease and stroke. Monitoring blood pressure in units of mmHg is vital for early detection and treatment of such conditions.
2. Air Pressure in Weather Systems
Atmospheric pressure influences weather patterns and is a key component in meteorology. Low-pressure systems are often associated with stormy weather, while high-pressure systems usually bring clear skies and calm conditions. Understanding how pressure affects weather helps meteorologists predict changes in weather patterns and issue warnings for severe weather conditions.
Example: Barometers measure atmospheric pressure in millibars or hectopascals. A significant drop in pressure indicates an approaching storm, while rising pressure typically signals improving weather.
3. Pressure in Fluids: Hydraulics
Hydraulic systems rely on the principle of pressure to transfer force through a fluid, usually oil or water. By applying pressure to a fluid in a confined space, hydraulic systems can generate significant mechanical force, which is used in machinery such as car brakes, construction equipment, and aircraft landing gear.
Example: Car brakes use hydraulic pressure to stop a vehicle. When you press the brake pedal, it applies pressure to a hydraulic fluid, which is transmitted to the brake pads, forcing them against the wheels to slow or stop the vehicle.
4. Pressure in Aviation
Pressure is a crucial factor in aviation, particularly in maintaining safe cabin conditions at high altitudes. As an airplane ascends, atmospheric pressure decreases, which can make it difficult to breathe. To address this, airplanes are equipped with pressurized cabins that maintain a comfortable air pressure inside the aircraft, even at altitudes where the outside air pressure is too low to support human life.
Example: At an altitude of 30,000 feet, the atmospheric pressure outside an airplane is much lower than at sea level. Without pressurization, the low pressure would make it difficult for passengers to breathe and could lead to altitude sickness or other health issues.
Pressure and the Laws of Gases
The relationship between pressure, volume, and temperature is governed by fundamental laws of physics known as the gas laws. Two important gas laws that describe the behavior of gases under varying conditions are Boyle’s Law and Charles’s Law.
- Boyle’s Law:
Boyle’s Law states that, for a fixed amount of gas at constant temperature, the pressure of a gas is inversely proportional to its volume. In other words, if you decrease the volume of a gas, its pressure increases, and vice versa.P1V1=P2V2Example: In a syringe, when the plunger is pushed, the volume inside the syringe decreases, causing the pressure to increase, forcing the fluid out. - Charles’s Law:
Charles’s Law states that, for a fixed amount of gas at constant pressure, the volume of a gas is directly proportional to its temperature. As the temperature increases, the gas molecules move faster, causing the gas to expand. Example: Hot air balloons rise because the air inside the balloon is heated, increasing its volume. This makes the balloon less dense than the surrounding cooler air, allowing it to float.
Example: Hot air balloons rise because the air inside the balloon is heated, increasing its volume. This makes the balloon less dense than the surrounding cooler air, allowing it to float.
Conclusion
Pressure is a vital concept that affects a wide range of fields, from biology and weather forecasting to engineering and aviation. By understanding the units of pressure, such as pascals, atmospheres, bars, torrs, and psi, we can accurately measure and apply pressure in various real-world contexts. Whether it’s monitoring blood pressure for health, maintaining tire pressure for vehicle safety, or using pressure in hydraulic systems to move heavy loads, pressure plays an essential role in the physical world.
Grasping the principles of pressure not only helps in understanding everyday phenomena but also opens the door to exploring more advanced scientific and industrial applications.