Valence Bond Theory (VBT) is a fundamental concept in chemistry that describes how atoms bond by overlapping their atomic orbitals to form a molecule. Developed in the early 20th century, VBT provides insights into the nature of chemical bonds, particularly covalent bonds, where atoms share electron pairs to achieve stable configurations. While VBT has been largely complemented by Molecular Orbital Theory (MOT) for more complex bonding scenarios, it remains valuable for understanding simple bonding in molecules.
This article explores the basics of Valence Bond Theory, its principles, bonding mechanisms, examples, and its applications, providing a foundational understanding of how atoms bond in molecules.
What is Valence Bond Theory?
Valence Bond Theory, proposed by Linus Pauling and others, explains how covalent bonds form between atoms by the overlap of atomic orbitals containing valence electrons. According to VBT, when two atoms come close to each other, their atomic orbitals overlap, allowing their electrons to pair and create a bond. The resulting covalent bond stabilizes the atoms by achieving a full outer electron shell, which corresponds to a lower energy state for the molecule.
VBT focuses on electron pairing in localized atomic orbitals and emphasizes the idea of bond formation through orbital overlap. This approach helps explain the geometry and bond angles of molecules based on the types of orbitals involved in bonding.
Key Concepts of Valence Bond Theory
1. Atomic Orbital Overlap: Bonds form when the orbitals of two atoms overlap, allowing electrons to be shared between atoms.
2. Localized Bonding: Unlike Molecular Orbital Theory, which describes delocalized electrons, VBT describes bonds as localized between specific pairs of atoms.
3. Bond Strength: Greater orbital overlap leads to stronger bonds. For example, a sigma (σ) bond, which involves head-to-head overlap, is typically stronger than a pi (π) bond, which involves side-by-side overlap.
4. Hybridization: VBT introduces the concept of orbital hybridization, where atomic orbitals combine to form new, hybrid orbitals that can form stronger bonds and create specific geometries.
Principles of Valence Bond Theory
Valence Bond Theory relies on several key principles to explain how atoms bond in molecules:
1. Atomic Orbital Overlap
The core idea of VBT is that covalent bonds form when atomic orbitals overlap. Each atom in a molecule contributes an orbital containing a single, unpaired electron, allowing these electrons to pair up and create a stable bond. The extent of orbital overlap affects bond strength, with greater overlap resulting in stronger bonds.
Example: In the hydrogen molecule (![]() ), each hydrogen atom has a 1s orbital containing one unpaired electron. When the two hydrogen atoms approach each other, their 1s orbitals overlap, allowing the electrons to pair up and form a covalent bond. This overlap of the 1s orbitals creates a sigma (σ) bond, which is the primary type of covalent bond in VBT.
), each hydrogen atom has a 1s orbital containing one unpaired electron. When the two hydrogen atoms approach each other, their 1s orbitals overlap, allowing the electrons to pair up and form a covalent bond. This overlap of the 1s orbitals creates a sigma (σ) bond, which is the primary type of covalent bond in VBT.
2. Types of Bonds: Sigma (σ) and Pi (π) Bonds
VBT identifies two main types of bonds based on how atomic orbitals overlap:
- Sigma (σ) Bond: A sigma bond forms from the head-to-head overlap of orbitals along the internuclear axis (the line connecting the two nuclei). Sigma bonds are typically stronger than pi bonds due to the direct overlap of orbitals, which maximizes bonding interactions.
Example: In methane (![]() ), each carbon-hydrogen bond is a sigma bond formed by the overlap of carbon’s hybridized sp³ orbitals with the 1s orbitals of hydrogen atoms.
), each carbon-hydrogen bond is a sigma bond formed by the overlap of carbon’s hybridized sp³ orbitals with the 1s orbitals of hydrogen atoms.
- Pi (π) Bond: A pi bond forms from the side-by-side overlap of p orbitals above and below the internuclear axis. Pi bonds are generally weaker than sigma bonds because the overlap is less direct.
Example: In ethene (![]() ), the carbon-carbon double bond consists of one sigma bond and one pi bond. The sigma bond results from sp² hybrid orbital overlap, while the pi bond arises from the side-by-side overlap of unhybridized p orbitals on each carbon atom.
), the carbon-carbon double bond consists of one sigma bond and one pi bond. The sigma bond results from sp² hybrid orbital overlap, while the pi bond arises from the side-by-side overlap of unhybridized p orbitals on each carbon atom.
3. Hybridization of Atomic Orbitals
One of VBT’s significant contributions is the concept of hybridization, which helps explain molecular geometry and bond angles. Hybridization is the mixing of atomic orbitals (such as s and p orbitals) on an atom to form new hybrid orbitals with specific shapes and orientations. These hybrid orbitals can then overlap with orbitals from other atoms to form covalent bonds.
The type of hybridization depends on the number of electron pairs around the central atom:
- sp Hybridization: Linear geometry with a bond angle of 180°, involving one s and one p orbital. Found in molecules like carbon dioxide (
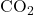 ) and acetylene (
) and acetylene (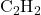 ).
). - sp² Hybridization: Trigonal planar geometry with a bond angle of 120°, involving one s and two p orbitals. Found in molecules like boron trifluoride (
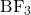 ) and ethene (
) and ethene (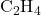 ).
). - sp³ Hybridization: Tetrahedral geometry with a bond angle of 109.5°, involving one s and three p orbitals. Found in molecules like methane (
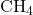 ) and ammonia (
) and ammonia (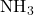 ).
).
Example: In methane (![]() ), the carbon atom undergoes sp³ hybridization, resulting in four sp³ hybrid orbitals. Each of these hybrid orbitals overlaps with a hydrogen 1s orbital, forming four sigma bonds and giving methane a tetrahedral structure.
), the carbon atom undergoes sp³ hybridization, resulting in four sp³ hybrid orbitals. Each of these hybrid orbitals overlaps with a hydrogen 1s orbital, forming four sigma bonds and giving methane a tetrahedral structure.
Examples of Valence Bond Theory in Action
To illustrate the application of VBT, let’s examine a few examples of common molecules and how VBT explains their bonding and structure.
1. Formation of Hydrogen Molecule (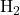 )
)
In the hydrogen molecule, each hydrogen atom has one unpaired electron in its 1s orbital. As the two hydrogen atoms approach each other, their 1s orbitals overlap, and the electrons pair up to form a covalent bond. This overlap creates a sigma bond along the internuclear axis, providing a stable arrangement with lower energy.
- Bond Type: Sigma (σ) bond due to 1s–1s orbital overlap.
- Bond Strength: Strong bond due to effective overlap.
- Bond Energy: Approximately 436 kJ/mol, which stabilizes the hydrogen molecule.
2. Formation of Methane (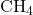 )
)
Methane consists of a central carbon atom bonded to four hydrogen atoms. Carbon has four valence electrons, but these are initially in 2s and 2p orbitals with different energies. To form four equivalent bonds, carbon undergoes sp³ hybridization, mixing one 2s and three 2p orbitals to create four sp³ hybrid orbitals with equal energy.
Each sp³ hybrid orbital overlaps with the 1s orbital of a hydrogen atom, forming four sigma bonds arranged in a tetrahedral geometry with bond angles of 109.5°.
- Hybridization: sp³
- Bond Type: Four sigma bonds (C–H)
- Geometry: Tetrahedral, with bond angles of 109.5°
3. Formation of Ethene (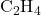 )
)
Ethene has a carbon-carbon double bond, with each carbon atom also bonded to two hydrogen atoms. Each carbon atom in ethene undergoes sp² hybridization, forming three sp² hybrid orbitals and one unhybridized p orbital.
- Sigma Bonds: The sp² orbitals of each carbon overlap with the sp² orbital of the other carbon and with the 1s orbitals of hydrogen atoms, forming sigma bonds.
- Pi Bond: The unhybridized p orbitals on each carbon atom overlap side-by-side to form a pi bond.
- Geometry: Trigonal planar with a bond angle of 120° around each carbon atom.
4. Formation of Acetylene (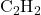 )
)
In acetylene, each carbon atom forms a triple bond with the other carbon and a single bond with hydrogen. Each carbon atom undergoes sp hybridization, creating two sp hybrid orbitals and leaving two unhybridized p orbitals.
- Sigma Bond: One sp orbital from each carbon overlaps to form a sigma bond along the internuclear axis.
- Pi Bonds: The two unhybridized p orbitals on each carbon overlap side-by-side, creating two pi bonds.
- Geometry: Linear with a bond angle of 180°.
Applications of Valence Bond Theory
VBT is widely used to explain bonding in simple organic and inorganic molecules, and it provides valuable insights in areas such as:
1. Predicting Molecular Shape and Bond Angles
VBT, along with hybridization, helps predict the three-dimensional shape of molecules and bond angles. By determining the type of hybrid orbitals involved, VBT can describe geometries such as linear, trigonal planar, and tetrahedral, essential for understanding molecular structure and reactivity.
- Example: In ammonia (
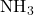 ), the nitrogen atom undergoes sp³ hybridization, resulting in a trigonal
), the nitrogen atom undergoes sp³ hybridization, resulting in a trigonal
pyramidal shape with bond angles of approximately 107°.
2. Explaining Bonding in Organic Compounds
VBT is particularly useful in organic chemistry, where it explains the bonding and structure of hydrocarbons, alcohols, acids, and other compounds with covalent bonds. The theory’s emphasis on sigma and pi bonds is essential for understanding double and triple bonds in organic molecules.
- Example: In ethyne (
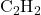 ), VBT describes the bonding structure as involving one sigma bond and two pi bonds in the carbon-carbon triple bond.
), VBT describes the bonding structure as involving one sigma bond and two pi bonds in the carbon-carbon triple bond.
3. Catalysis and Transition Metal Complexes
VBT, particularly when combined with hybridization, helps in the study of transition metal complexes, where transition metals form bonds with ligands through d orbital overlap. Understanding how orbitals overlap in these complexes is essential for designing catalysts in industrial and biochemical processes.
- Example: In coordination compounds like
![Rendered by QuickLaTeX.com \text{[Cu(NH}_3\text{)}_4\text{]}^{2+}](https://www.sridianti.com/wp-content/uploads/2024/11/quicklatex.com-fd5e1c9664c1018cd22b9a2c7855a510_l3.png) , VBT explains the bonding by considering the hybridization of the metal’s d orbitals to accommodate bonding with ligands.
, VBT explains the bonding by considering the hybridization of the metal’s d orbitals to accommodate bonding with ligands.
4. Explaining Magnetic Properties
VBT can also explain the magnetic properties of certain compounds, particularly when describing unpaired electrons in molecular structures. Compounds with unpaired electrons exhibit paramagnetic properties, while those with paired electrons are diamagnetic.
- Example: In molecular oxygen (
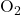 ), VBT accounts for the unpaired electrons that contribute to its paramagnetic behavior.
), VBT accounts for the unpaired electrons that contribute to its paramagnetic behavior.
Limitations of Valence Bond Theory
While VBT has contributed significantly to our understanding of chemical bonding, it has certain limitations:
1. Delocalized Bonding: VBT struggles to explain delocalized bonding, as seen in molecules like benzene, where electrons are shared across multiple atoms.
2. Inadequate for Complex Molecules: VBT’s localized bonding approach limits its effectiveness in describing large or complex molecules, where electron delocalization and resonance are important.
3. Does Not Explain Spectroscopic Properties: Unlike Molecular Orbital Theory, VBT does not provide information about the energy levels of electrons in different molecular orbitals.
Conclusion
Valence Bond Theory remains a foundational concept in chemistry, explaining how atoms bond through orbital overlap to form stable molecules. By emphasizing sigma and pi bonds, hybridization, and localized bonding, VBT provides insights into the structure and geometry of molecules. Despite its limitations, VBT is instrumental in understanding simple molecules, bonding in organic compounds, and even transition metal complexes. As a stepping stone to more advanced theories, Valence Bond Theory has laid the groundwork for modern approaches in chemistry, allowing chemists to predict and manipulate molecular structures in both theoretical and practical applications.